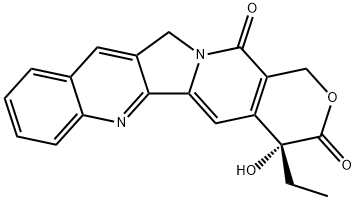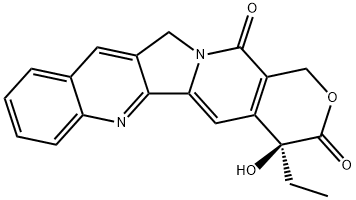
(+)-Camptothecin synthesis
- Product Name:(+)-Camptothecin
- CAS Number:7689-03-4
- Molecular formula:C20H16N2O4
- Molecular Weight:348.35
Yield:37 % ee
Reaction Conditions:
Stage #1: 4-ethyl-1,12-dihydro-14-H-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14-(4H)-dionewith (+)-[(8,8-dimethoxycamphoryl)sulfonyl]oxaziridine;potassium hexamethylsilazane in tetrahydrofuran;dichloromethane at 20; for 19 h;
Stage #2: with waterOverall yield = 32 %; Overall yield = 5 mg;
Steps:
(S)-4-Ethyl-4-hydroxy-1,12-dihydro-14H-pyrano[3',4':6,7]-indolizino[1,2-b]quinoline-3,14(4H)-dione ((+)-camptothecin (1a)).
KHMDS (0.5 M in toluene, 100 μl, 49.6 μmol,1.1 equiv) was added dropwise to a cold (-78°C) solution of 4-ethyl-1,12-dihydro-14H-pyrano[3',4':6,7]indolizino[1,2-b]-quinoline-3,14(4H)-dione (25) (15.0 mg, 45.1 μmol, 1.0 equiv) in CH2Cl2 (750 μl). Subsequently, (+)-[(8,8-dimethoxycamphoryl)sulfonyl]oxaziridine (30.0 mg, 0.104 mmol,2.3 equiv) in THF (750 μl) was added and the reaction mixture was allowed to warm to room temperature over 19 h. Saturated aqueous NH4Cl solution (25 ml) was added, and the aqueous phase was extracted with EtOAc (3×25 ml). The combined organic layers were washed with saturated aqueous Na2SO3 solution (25 ml) and saturated aqueous NaCl solution (25 ml), dried over anhydrous MgSO4, andthe solvent was removed under reduced pressure. Silica gelcolumn chromatography (CH2Cl2-MeOH, 100:1) providedthe title compound 1. Yield 5 mg, (32%), pale-yellow solid.[α]D20 +14.4° (c 0.34, CHCl3-MeOH, 4:1). HPLC(Chiralpak IA, (n-hexane-MeOH-CH2Cl2 4:2:1)-CH2Cl2,80:20, flow rate 0.6 ml/min, 254 nm): tR 13.7 min (R)-(1),tR14.6 min (S)-(1), 37% ee. ([α]D20 +14.2° (c 0.34, CHCl3-MeOH, 4:1)13b). The remaining analytical data correspondto the racemic compound.
References:
Tietze, Lutz F.;Bischoff, Matthias;Khan, Taukeer A.;Liu, Deshan [Chemistry of Heterocyclic Compounds,2017,vol. 53,# 4,p. 434 - 445][Khim. Geterotsikl. Soedin.,2017,vol. 53,# 4,p. 434 - 445,12]
![1H-Pyrano[3,4-c]pyridine-3,8(4H,7H)-dione, 7-[(2-bromo-3-quinolinyl)methyl]-4-ethyl-4-hydroxy-, (4S)-](/CAS/20210305/GIF/146596-04-5.gif)
146596-04-5
0 suppliers
inquiry

7689-03-4
564 suppliers
$18.00/1g
![14H-Pyrano[3',4':6,7]indolizino[1,2-b]quinolin-14-one, 4-ethyl-1,12-dihydro-](/CAS/20210305/GIF/841276-70-8.gif)
841276-70-8
0 suppliers
inquiry

7689-03-4
564 suppliers
$18.00/1g
![14H-Pyrano[3',4':6,7]indolizino[1,2-b]quinolin-14-one, 4-ethyl-1,3,4,12-tetrahydro-3,4-dihydroxy-, (4S)-](/CAS/20210305/GIF/210563-62-5.gif)
210563-62-5
0 suppliers
inquiry

7689-03-4
564 suppliers
$18.00/1g
![1H-Pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione, 4-ethyl-](/CAS/20210305/GIF/34141-35-0.gif)