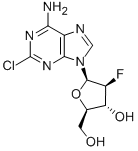
Clofarabine synthesis
- Product Name:Clofarabine
- CAS Number:123318-82-1
- Molecular formula:C10H11ClFN5O3
- Molecular Weight:303.68

Cen, Yana; Sauve, Anthony A. Efficient Syntheses of Clofarabine and Gemcitabine From 2-Deoxyribonolactone. Nucleosides, Nucleotides & Nucleic Acids. Department of Pharmacology. Weill Medical College of Cornell University. New York, USA. Volume 29. Issue 2. Pages 113-122. 2010
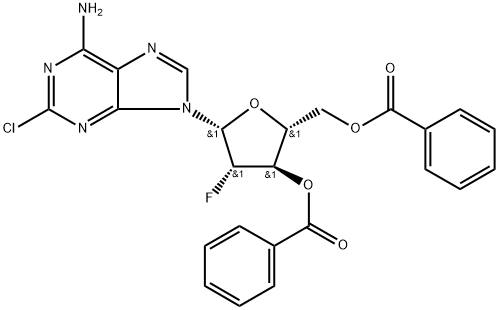
355138-50-0
8 suppliers
inquiry

123318-82-1
394 suppliers
$7.00/10mg
Yield:123318-82-1 95.41%
Reaction Conditions:
with methanol;sodium methylate at 15 - 20; for 2 h;
Steps:
2
The last step intermediate obtained clofarabine added 10mL of anhydrous methanol,After stirring, NaOCH3 / CH3OH solution (193.41mg dissolved in 2mL anhydrous methanol) was added, and the mixture was stirred at 15-20 for 2h. The reaction was complete by TLC. After completion of the reaction, the insoluble matter was removed by filtration, and a large amount of solid was precipitated from the filtrate. The pH was adjusted to 6.5 to 7.5 by adding acetic acid under stirring. After adjustment, the mixture was stirred at room temperature for 30 minutes, filtered and the cake was washed with a small amount of cold methanol to obtain clofarabine, which was vacuum dried at 50C to obtain 1.73 g of clofarabine product in a yield of 95.41%. Result of liquid phase: 99.85%.
References:
CN105601688,2016,A Location in patent:Paragraph 0049- 0058

1234346-14-5
1 suppliers
inquiry

123318-82-1
394 suppliers
$7.00/10mg
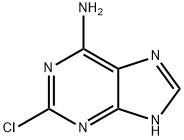
1839-18-5
345 suppliers
$10.00/1g

69123-94-0
144 suppliers
$60.00/1g

123318-82-1
394 suppliers
$7.00/10mg
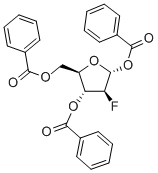
97614-43-2
322 suppliers
$5.00/250mg

123318-82-1
394 suppliers
$7.00/10mg
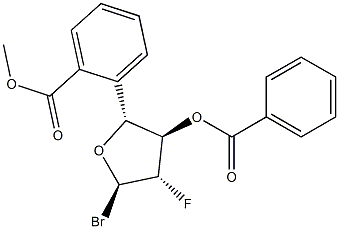
97614-44-3
58 suppliers
$45.00/100mg

123318-82-1
394 suppliers
$7.00/10mg







