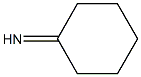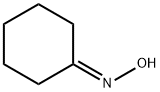
Cyclohexanone oxime synthesis
- Product Name:Cyclohexanone oxime
- CAS Number:100-64-1
- Molecular formula:C6H11NO
- Molecular Weight:113.16
The partial reduction of aliphatic nitro compounds was mentioned as early as the turn of this century. However, only with the commercialization of nitroalkanes did these reactions achieve any real significance. Among the chemical reducing agents, zinc dust and acetic acid have been recommended.
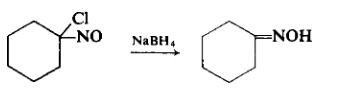
Hydrogenation of nitrocyclohexane on a silver dichromate catalyst has recently been patented. In this procedure, it is said to be important to control the hydrogen take-up to prevent hydrogenation of the oxime to the hydroxylamine. This is accomplished by venting hydrogen off as soon as the theoretical quantity of hydrogen has been used up to convert the nitro compound to the oxime.
Olefinic nitro compounds have been reduced to the saturated oxime with hydrogen and a palladium-on-carbon catalyst. To maximize the yield of oxime, 0.5-1.0 mole of hydrogen chloride per mole of nitroolefin must be present. Since the by-products contain crude ketones also, a posttreatment with hydroxylamine hydrochloride and sodium acetate has been recommended. By this means, 1-nitrocyclooctene has been converted to cyclooctanone oxime [b.p. 63°C (0.08 mm Hg), m.p. 41.7-42.7°C] and 1-nitro-l-octadecene has been converted to stearaldoxime (m.p. 88-89.8°C). Whether this method is confined to 1-olefin derivatives is not clear.
Nitro olefins have also been reduced with zinc dust and acetic acid, to produce oximinoalkanes. The preparation of 5-ethyl-3-nonanone oxime gives the necessary details. To be noted is that the carbon bearing nitro group in the starting material also bears the double bond. Whether this structural feature is essential if the reduction is to stop at the oxime stage may need further elucidation.
Yield:100-64-1 83.5%
Reaction Conditions:
with Cumene hydroperoxide;ammonia;Ti-MWW in acetonitrile at 115; under 3750.38 Torr; for 1 h;Product distribution / selectivity;
Steps:
4
Example 4In a 1 L autoclave (reactor), 159.4 g of an acetonitrile solution containing 2.3% by weight of ammonia, 7.6 g of a cumene solution containing 80% by weight of cumene hydroperoxide and 5.0 g of Ti-MWW (prepared by the same manner as described in Chemistry Letters, 2000, pp. 774-775) were charged and a vapor phase portion in the reactor was replaced by nitrogen. After the reactor was sealed, the temperature in the reactor was raised to 115° C. under stirring. The pressure in the reactor was 0.5 MPa. Next, 10 g of an acetonitrile solution containing 4.7% by weight of cyclohexanone, and 115 g of an acetonitrile solution containing 3.8% by weight of ammonia and 1.0% by weight of cumene hydroperoxide each were fed (co-fed) to the reactor over 1 hour. The concentration of ammonia in the liquid phase of the reaction mixture changed within a range from 1.0 to 2.3% by weight relative to the liquid phase.After the co-feeding, the liquid phase of the reaction mixture was withdrawn and analyzed by gas chromatography. The conversion rate of cyclohexanone was found 99.5%, the selectivity to cyclohexanone oxime was 83.9% and the yield of cyclohexanone oxime was 83.5%. Selectivity to cyclohexanoneimine (a compound produced by imination of cyclohexanone) and impurities derived from imine, based on the consumed cyclohexanone, was 16.0%.
References:
SUMITOMO CHEMICAL COMPANY, LIMITED US2010/16637, 2010, A1 Location in patent:Page/Page column 3

108-94-1
524 suppliers
$12.00/50g

100-64-1
272 suppliers
$5.00/25g
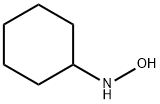
2211-64-5
53 suppliers
$27.00/100mg

100-64-1
272 suppliers
$5.00/25g

13871-89-1
1 suppliers
inquiry

100-64-1
272 suppliers
$5.00/25g