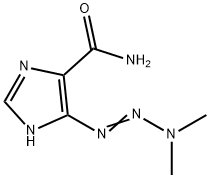
Dacarbazine synthesis
- Product Name:Dacarbazine
- CAS Number:4342-03-4
- Molecular formula:C6H10N6O
- Molecular Weight:182.18
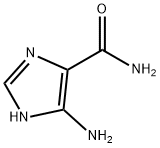
360-97-4
363 suppliers
$5.00/250mg
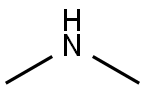
124-40-3
499 suppliers
$18.00/100ml

4342-03-4
299 suppliers
$5.00/50mg
Yield:4342-03-4 76.2%
Reaction Conditions:
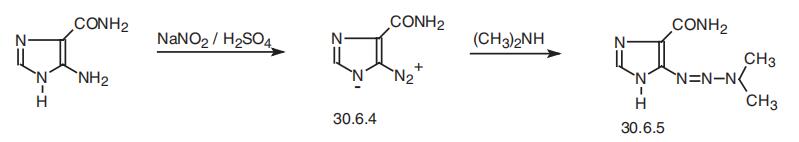
Stage #1:5-Aminoimidazole-4-carboxamide with hydrogenchloride;sodium nitrite in water for 0.5 h;Cooling with ice;
Stage #2:dimethyl amine in water for 2 h;Cooling with ice;
Steps:
The synthesis of dacarbazine is as follows:
take 378 mg (3.0 mmol) of 5-amino-4-imidazolecarboxamide in a 50 mL round bottom flask, add 1.0 mL of distilled water and stir in an ice bath; take 1.2 mL (about 12.0 mmol) of concentrated hydrochloric acid and use After diluting with 2.0mL of water, it was slowly added to the reaction flask. The reaction was always carried out in an ice bath. 207mg (3.0mmol) of sodium nitrite (NaNO2) was dissolved in 1.0mL of distilled water and slowly added to the reaction system. Potassium starch iodide Test strips confirmed the excess of sodium nitrite during the reaction. At this time, the reaction solution turned dark red, and the reaction was always performed under an ice bath. After stirring for 0.5 h, 0.5 mL (4.5 mmol) of an aqueous solution containing 40% dimethylamine was added dropwise to the reaction system, and the reaction was continued at a low temperature for 2 h. After completion of the reaction, the reaction solution was lyophilized. The lyophilized residue was dissolved in water and passed through a solid-phase chromatography column and a 0.25 μm filter membrane. The sample was purified by RP-HPLC [eluent: V (methanol): V (water) = 15: 85]. The fractions were combined and the temperature was low. The methanol was removed by rotary evaporation, and the residual liquid was lyophilized to obtain 415 mg of a pale red solid powder with a yield of 76.2%.
References:
Suzhou Lanyun Pharmaceutical Technology Co., Ltd.;Jin Wei;Liu Jinlun;Wang Jianbing CN110305067, 2019, A Location in patent:Paragraph 0034; 0035