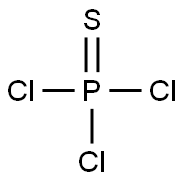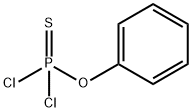
Dichlorophenoxysulfanylidene phosphorane synthesis
- Product Name:Dichlorophenoxysulfanylidene phosphorane
- CAS Number:18961-96-1
- Molecular formula:C6H5Cl2OPS
- Molecular Weight:227.05
Yield:-
Reaction Conditions:
with triethylamine in dichloromethane at -78 - 20; for 16 h;
Steps:
9
A solution of phenol (15 g) in dichloromethane (DCM) was added to a cold (-78° C.) solution of phosphorothioyl trichloride 26 (16.13 mL) in DCM followed by addition of triethylamine (TEA, 22 mL). After the addition was complete the solution was warmed to room temperature and stirred overnight (16 h). DCM was evaporated and the residue triturated by methyl tert-butylether (MTBE). The solid (triethylamine hydrochloride) was filtered off and the filtrate evaporated to dryness. The residue, O-phenylphosphorodichloridothioate 27, was used without purification in the next step.
References:
Achillion Pharmaceuticals, Inc.;Deshpande, Milind;Wiles, Jason Allan;Hashimoto, Akihiro;Phadke, Avinash US2014/309164, 2014, A1 Location in patent:Paragraph 0297; 0298

108-95-2
717 suppliers
$14.00/25g

18961-96-1
22 suppliers
inquiry
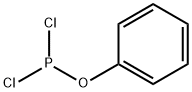
3426-89-9
22 suppliers
inquiry

18961-96-1
22 suppliers
inquiry