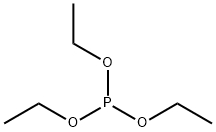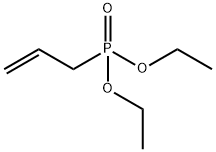
DIETHYL ALLYLPHOSPHONATE synthesis
- Product Name:DIETHYL ALLYLPHOSPHONATE
- CAS Number:1067-87-4
- Molecular formula:C7H15O3P
- Molecular Weight:178.17
Yield:1067-87-4 98%
Reaction Conditions:
at 71; for 3 h;Inert atmosphere;Michaelis-Arbuzov Synthesis;
Steps:
Diethyl (prop-2-en-1-yl)phosphonate
To a round-bottomed flask equipped with a reflux condenser and under a nitrogen atmosphere freshly distilled triethyl phosphite (5.16 mL, 30 mmol) and allyl bromide (5.52 mL, 33 mmol) was added. The mixture was heated to 71 °C, 3 h. Then the excess of allyl bromide was distilled off by heating the crude mixture in the range of about 120 °C for 2 h. Ester (1) as a colourless oil (5.25 g, 98%) was obtained. The purity of the product was determined by TLC and GC. Their physical and spectral data are as follows:TLC Rf = 0.41 (CHCl3:MeOH 45:1 v/v); GC Rt = 4.02 min; 1H NMR (600 MHz, CDCl3) δ: 1.30 (t, J = 7.1 Hz, 6H, 2x -OCH2CH3), 2.60 (ddt, J = 21.9, 7.4, 1.2 Hz, 2H, CH2-P), 4.14 - 4.04 (m, 4H, 2x -OCH2CH3), 5.23-5.16 (m, 2H, CH=CH2), 5.78 (m, 1H, CH=CH2); 13C NMR (151 MHz, CDCl3) δ: 16.44 and 16.40 (-OCH2CH3), 32.25 (d, J = 138.3 Hz, CH2-P), 61.95 and 61.90 (-OCH2CH3), 119.95 (d, J = 14.4 Hz, =CH2), 127.56 (d, J = 11.2 Hz, =CH-); 31P NMR (243 MHz, CDCl3) δ: 27.08; EIMS (%): 41.2 (17.5), 65.2 (17.5), 81.2 (33.7), 91.2 (22.1), 97.1 (31.2), 109.1 (99.9), 123.2 (27.4), 134.0 (38.5), 151,1 (31.7), 178.8 [M+] (89.9).
References:
Mitula, Pawel;Wawrzenczyk, Czeslaw [ARKIVOC,2012,vol. 2012,# 4,p. 216 - 232]

106-95-6
416 suppliers
$10.00/5g
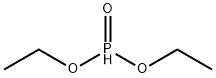
762-04-9
318 suppliers
$10.00/10 g

1067-87-4
106 suppliers
$14.00/1g
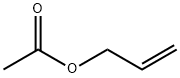
591-87-7
177 suppliers
$10.00/10g

762-04-9
318 suppliers
$10.00/10 g

1067-87-4
106 suppliers
$14.00/1g

1469-70-1
73 suppliers
$60.00/100mg

762-04-9
318 suppliers
$10.00/10 g

1067-87-4
106 suppliers
$14.00/1g
1809-20-7
174 suppliers
$8.00/10g

106-95-6
416 suppliers
$10.00/5g

1067-87-4
106 suppliers
$14.00/1g