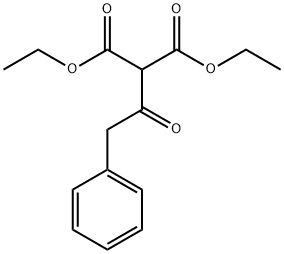
Diethyl(phenylacetyl)malonate synthesis
- Product Name:Diethyl(phenylacetyl)malonate
- CAS Number:20320-59-6
- Molecular formula:C15H18O5
- Molecular Weight:278.3
Yield:-
Reaction Conditions:
for 3 h;Reflux;
Steps:
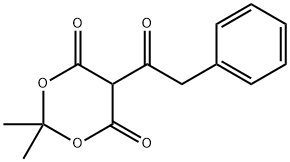
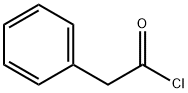
To a solution of Meldrum's acid (1.0 g, 7.0 mmol) and dry TEA (5 mL, 35.6 mmol) in dry dichloromethane (15 mL) was added phenylacetyl chloride (0.95 mL, 7.13 mmol) dropwise at ice bath temperature.
The reaction mixture was allowed to back to RT, stirred for 3.5 h.
Crude adduct of Meldrum's acid and acetyl chloride was washed by 3N hydrochloric acid and concentrated.
To the residue was added ethanoi (20 mL); the reaction mixture was heated to reflux for 3 h.
Resultant residue was made to react to N,N-dimethylformamide dimethyl acetal (1.5 mL, 11.2 mmol) in xylenes (15 mL) at 120° C. for 4 hrs with removal of methanol and concentrated in vacuo.
The residue was cyclized by ammonium acetate (1.15 g) in methanol (20 mL) at reflux for 4 hrs.
Crude ethyl ester was saponifieated by 10% sodium hydroxide solution (10 mL) in methanol (20 mL).
Reaction mixture was heated at 65° C. for 2.5 hours.
Solid precipitated during acidic workup by 3N hydrochloric acid was collected by filter, washed by methanol (small amount), water and diethyl ether, dried to give the title compound D125 (671.2 mg, 21% over four steps).
1H-NMR (400 MHz, DMSO-d6): 13.16 (brs, 1H, NH), 8.60 (d, J=1.6 Hz, 1H, pyridone-H), 8.23 (d, J=1.6 Hz, 1H, pyridone-H), 7.65 (d, J=8 Hz, 2H, ArH), 7.50-7.41 (m, 3H, ArH).
References:
US2013/281451,2013,A1 Location in patent:Paragraph 0283

570-08-1
120 suppliers
$30.00/5g

20320-59-6
93 suppliers
inquiry

137786-67-5
0 suppliers
inquiry

20320-59-6
93 suppliers
inquiry
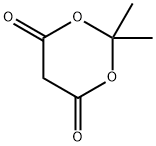
2033-24-1
662 suppliers
$6.00/25g

20320-59-6
93 suppliers
inquiry