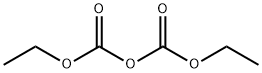
Diethyl pyrocarbonate synthesis
- Product Name:Diethyl pyrocarbonate
- CAS Number:1609-47-8
- Molecular formula:C6H10O5
- Molecular Weight:162.14
Yield:-
Reaction Conditions:
with sodium hydroxide;magnesium sulfate;PEG 15 stearamine in water
Steps:
1 EXAMPLE 1
EXAMPLE 1 This example describes the preparation of diethyl pyrocarbonate with PEG 15 stearamine as the catalyst. A solution of ethyl chloroformate (114.2 grams (95%) 1.00 mole) and PEG-15 stearamine (1.88 grams (99%) 0.002 mole) was introduce to a 1000 milliliter, three-necked reaction flask with a bottom stopcock. The reaction flask was equipped with a mechanical teflon blade stirrer, sodium hydroxide solution inlet, vent and thermowell for a thermocouple, which was connected to a temperature control unit. Sodium hydroxide was charged to the reaction flask through a Masterflex addition pump. The temperature control unit activated a cooling water pump which directed ice water against the reaction flask and also controlled the Masterflex pump so as to maintain the reaction temperature at below 15° C. When the temperature in the reaction flask was below 15° C., sodium hydroxide solution was charged to the flask. When the temperature rose above 15° C., the Masterflex pump was shut off and ice water sprayed on the reaction flask to cool the contents of the reaction flask. Aqueous sodium hydroxide solution (82 grams (50%) 1.025 moles) was charged to the reaction flask over a period of approximately 60 minutes. Semi-solids which remained on the sides of the flask were rinsed into the liquid reaction mixture with a small amount of water and the reaction mixture post stirred at 15° C. for 30 minutes. 125.5 grams of water (including the amount of rinse water) was added tot he reaction flask so that the theoretical percent solids in the aqueous phase was about 25 percent. The reaction mixture was agitated from 10 seconds to dissolve all solids before phase separation was performed. The top organic phase (82.7 grams) was dried over 6.2 grams of magnesium sulfate to give a light yellow clear liquid (77.2 grams). Volatiles in the yellow liquid product were removed by a rotary evaporator under water aspirator vacuum (40 mm/Hg) for 60 minutes at 60° C., and then under vacuum pump (<2 mm/Hg) for 60 minutes at 60° C., thereby to obtain a final liquid product (71.8 grams). This product was a clear, light yellow liquid having an APHA color of 100.
References:
PPG Industries, Inc. US5231211, 1993, A
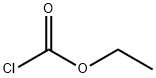
541-41-3
6 suppliers
$19.39/5g

1609-47-8
314 suppliers
$5.00/1g
