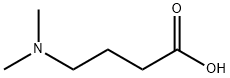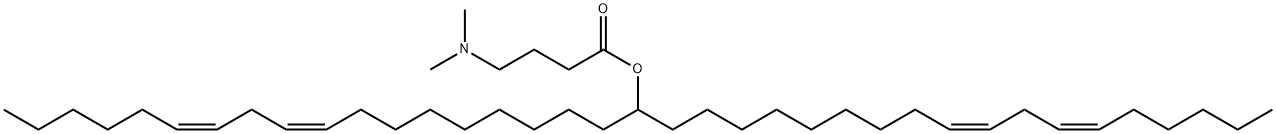
DLin-M-C3-DMA synthesis
- Product Name:DLin-M-C3-DMA
- CAS Number:1224606-06-7
- Molecular formula:C43H79NO2
- Molecular Weight:642.09
DLin-M-C3-DMA is useful for design of lipid nanoparticles for in vitro and in vivo delivery of plasmid DNA,it is synthesised by CIS,CIS-9,12-OCTADECADIENOL.

1169768-28-8
18 suppliers
inquiry
69954-66-1
111 suppliers
$44.50/5g

1224606-06-7
129 suppliers
inquiry
Yield:1224606-06-7 95%
Reaction Conditions:
with dmap;1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride;N-ethyl-N,N-diisopropylamine in dichloromethaneInert atmosphere;
Steps:
12
Dilinoleylmethanol (7.8 g, 14.9 mmol), dimethylaminobutyric acid hydrochloride (2.99 g, 17.8 mmol) and a stir bar were added to 500 mL RBF. The flask was flushed with nitrogen and anh. DCM (120 mL) added, followed by EDCI (3.6 g, 18.8 mmol), DIPEA (6.3 mL, 36.3 mmol) and DMAP (450 mg, 3.69 mmol). The reaction was stirred overnight. The reaction was diluted with DCM (300 mL) and washed with sat. NaHC03 (200 mL), water (300 mL) and sat. NaCL (200 mL). Each aq. wash was extracted once with DCM (50 mL). Organics were combined, dried (MgS04) and concentrated to yield a yellow oil with some crystalline matter. This was purified by chromatography (0-2% MeOH in CHC13) to yield Lin-MC3 as a pale yellow oil (9.0 g, 14.1 mmol, 95%).
References:
PROTIVA BIOTHERAPEUTICS, INC;HEYES, James;WOOD, Mark;MARTIN, Alan WO2011/141704, 2011, A1 Location in patent:Page/Page column 109

51154-39-3
20 suppliers
inquiry

1224606-06-7
129 suppliers
inquiry

506-43-4
157 suppliers
$13.00/100mg

1224606-06-7
129 suppliers
inquiry
60-33-3
562 suppliers
$9.00/5g

1224606-06-7
129 suppliers
inquiry