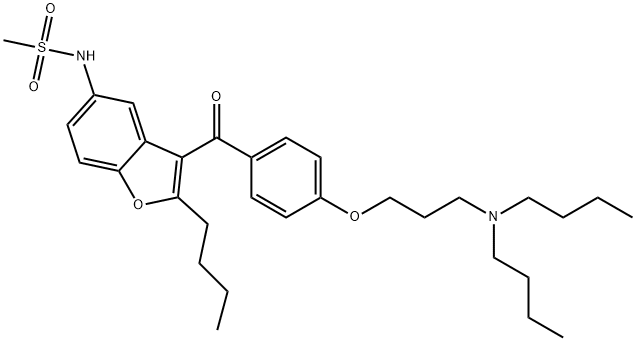
Dronedarone synthesis
- Product Name:Dronedarone
- CAS Number:141626-36-0
- Molecular formula:C31H44N2O5S
- Molecular Weight:556.76

Process for the preparation of dronedarone; Gutman, Arie L.; Nisnevich, Gennady; Yudovitch, Lev; Assignee ISP Investments Inc., USA 2003; Patent Information; May 15, 2003; WO 2003040120 A1
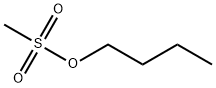
1912-32-9
135 suppliers
$6.00/1g
![Methanesulfonamide, N-[3-[4-(3-aminopropoxy)benzoyl]-2-butyl-5-benzofuranyl]-](/CAS/20210111/GIF/1026754-33-5.gif)
1026754-33-5
1 suppliers
inquiry

141626-36-0
166 suppliers
$50.00/10mM (in 1mL DMSO)
Yield:141626-36-0 79%
Reaction Conditions:
with pyridine in butan-1-ol at 70; for 14 h;
Steps:
2
0.8 g of N- [2-butyl-3 - { 4- [(3 -amino)propoxy] benzoyl } - 1 -benzofuran-5- yl]methanesulfonamide (II) is dissolved in 5 ml of n-butanol. 0.15 g of pyridine is added and the mixture is heated to 70 °C at this temperature 0.68 g of butyl-methanesulfohate (III) dissolved in 1 ml of n-butanol is added during 15 minutes and the mixture is kept at 70 °C for 14 hours. [The methanesulfonate is prepared according to the procedure described in J. of Steroid Biochemistry and Molecular Biology 80 (2002) 429-440.] The reaction mixture is evaporated and 20 ml of dichloromethane and 10 ml of water is added. The phases are separated. The organic phase is washed with 10ml of NaHC03 of 5 %. The organic phase is evaporated. The product is purified by on silica gel (ethyl acetate/hexane; 1 :3 v/v ).Yield: 0.79 g (79 %). Purity: 100 % (HPLC).The product is identical with the compound prepared in example 1.
References:
SANOFI;FRIESZ, Antal;PÁRKÁNYI, Zsolt;DOMBRÁDY, Zsolt WO2012/131409, 2012, A1 Location in patent:Page/Page column 14-15
![N-(2-butyl-3-[4-(3-oxopropoxy)benzoyl]-1-benzofuran-5-yl)methanesulfonamide](/CAS/20200401/GIF/1420180-98-8.gif)
1420180-98-8
1 suppliers
inquiry

111-92-2
384 suppliers
$14.00/25mL

141626-36-0
166 suppliers
$50.00/10mM (in 1mL DMSO)

1421316-69-9
1 suppliers
inquiry

141626-36-0
166 suppliers
$50.00/10mM (in 1mL DMSO)
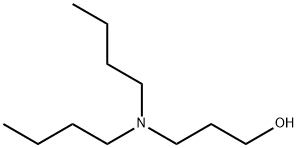
2050-51-3
51 suppliers
$62.50/250 mg
![Benzenesulfonic acid, 4-[(methylsulfonyl)amino]-, 4-[[2-butyl-5-[(methylsulfonyl)amino]-3-benzofuranyl]carbonyl]phenyl ester](/CAS/20210305/GIF/1448297-14-0.gif)
1448297-14-0
0 suppliers
inquiry

141626-36-0
166 suppliers
$50.00/10mM (in 1mL DMSO)








