ethyl-2,2-dimethyl-7-bromoheptanoate synthesis
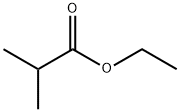
- Product Name:ethyl-2,2-dimethyl-7-bromoheptanoate
- CAS Number:123469-92-1
- Molecular formula:C11H21BrO2
- Molecular Weight:265.19
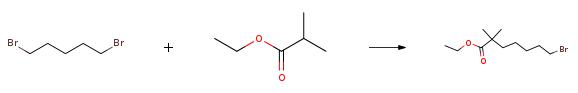
The specific embodiment of the present invention is as follows: prepare 19.4g (167mmol) of ethyl isobutyrate in 216mL solution of tetrahydrofuran with a mass concentration of 10%, connect to metering pump 1; prepare 84mL (168mmol) of 2.0M n-butyllithium solution, connect to meter Pump 2; configure 1,5-dibromopentane 80.0g (348mmol) tetrahydrofuran 100mL solution, mass concentration is 63%, connected to metering pump 3.Configure 300mL of 1N hydrochloric acid solution and connect to metering pump 4.Set continuous flow precooler 1, the circulating temperature is 0, and reach stability; set continuous flow precooler 2, the circulating temperature is 0, and reach stability; set continuous flow precooler 3, and the circulating temperature is 0, and reach stability; set continuous flow precooler 4, cycle temperature is 0, and reach stability;Set the flow rate of metering pump 1 to 65.0ml/min, set the flow rate of metering pump 2 to 25.0ml/min, set the flow rate of metering pump 3 to 30.0ml/min, and set the flow rate of metering pump 4 to 90.0ml/min.Turn on the metering pump 1 and metering pump 2 at the same time. After 60s of operation, turn on the metering pump 3, and after 60s of operation, turn on the metering pump 4 to collect the outflowing reaction liquid in the storage tank.After the operation is completed, separate the liquids, collect the upper organic phase, concentrate under reduced pressure at P=-0.08MPa, T=45°C to remove low-boiling solvents, and then rectify under reduced pressure at P=5mmHg and T=70°C to obtain 38.4 g.The obtained product is a colorless liquid with a GC purity of 98.6% and a yield of 87%.
Yield:123469-92-1 87%
Reaction Conditions:
with hydrogenchloride;n-butyllithium in tetrahydrofuran;water monomer at 0; for 0.0333333 h;Flow reactor;Reagent/catalyst;
Steps:
1-6 Example 6
The specific embodiment of the present invention is as follows: prepare 19.4g (167mmol) of ethyl isobutyrate in 216mL solution of tetrahydrofuran with a mass concentration of 10%, connect to metering pump 1; prepare 84mL (168mmol) of 2.0M n-butyllithium solution, connect to meter Pump 2; configure 1,5-dibromopentane 80.0g (348mmol) tetrahydrofuran 100mL solution, mass concentration is 63%, connected to metering pump 3.Configure 300mL of 1N hydrochloric acid solution and connect to metering pump 4.Set continuous flow precooler 1, the circulating temperature is 0, and reach stability; set continuous flow precooler 2, the circulating temperature is 0, and reach stability; set continuous flow precooler 3, and the circulating temperature is 0, and reach stability; set continuous flow precooler 4, cycle temperature is 0, and reach stability;Set the flow rate of metering pump 1 to 65.0ml/min, set the flow rate of metering pump 2 to 25.0ml/min, set the flow rate of metering pump 3 to 30.0ml/min, and set the flow rate of metering pump 4 to 90.0ml/min.Turn on the metering pump 1 and metering pump 2 at the same time. After 60s of operation, turn on the metering pump 3, and after 60s of operation, turn on the metering pump 4 to collect the outflowing reaction liquid in the storage tank.After the operation is completed, separate the liquids, collect the upper organic phase, concentrate under reduced pressure at P=-0.08MPa, T=45°C to remove low-boiling solvents, and then rectify under reduced pressure at P=5mmHg and T=70°C to obtain 38.4 g.The obtained product is a colorless liquid with a GC purity of 98.6% and a yield of 87%.
References:
CN111675614,2020,A Location in patent:Paragraph 0030; 0033-0075

111-24-0
389 suppliers
$5.00/5g

123469-92-1
131 suppliers
inquiry