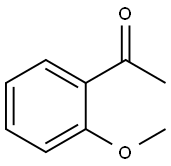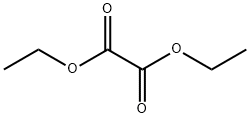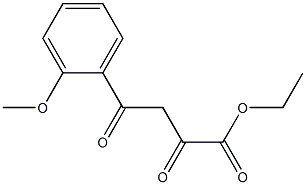
Ethyl 2-Methoxy-a,g-dioxo-benzenebutanoate synthesis
- Product Name:Ethyl 2-Methoxy-a,g-dioxo-benzenebutanoate
- CAS Number:60943-39-7
- Molecular formula:C13H14O5
- Molecular Weight:250.25
Yield:-
Reaction Conditions:
with sodium hydride in toluene at 80; for 15 h;
Steps:
Compound 25: 3-((2-fluorophenoxy)methyl)-5-(2-methoxyphenyl)-1H-pyrazole Referring to Scheme 3,2-methoxyacetophenone (4.5 g) was reacted with diethyl oxalate (8.2 ml) and sodium hydride (60% in oil; 3.6 g) in toluene (40 ml) at 80° C. for 15 hours. The reaction mixture was cooled to room temperature and then quenched with water. The mixture was acidified with 1N HCl and extracted with ethyl acetate. The extract was washed with brine, dried over Na2SO4, and concentrated in vacuo to give Compound 22. A solution of Compound 22 and hydrazine monohydrate (0.5 ml) in ethanol (10 ml) was stirred for 15 hours at room temperature. The reaction mixture was diluted with water extracted with ethyl acetate. The extract was washed with brine, dried over Na2SO4, concentrated in vacuo, and purified by silica gel chromatography to give Compound 23. To a suspension of calcium chloride (444 mg) and sodium borohydride (303 mg) in ethanol (5 ml) and THF (2 ml) was added a solution of Compound 23 (246 mg) in THF (3 ml) at 0° C. and the resulting mixture was stirred at 0° C. for 15 hours. The reaction mixture was acidified with 1N HCl and concentrated in vacuo. The residue was diluted with water and extracted with ethyl acetate. The extract was washed with brine, dried over Na2SO4, and concentrated in vacuo to give Compound 24. A solution of Compound 24 (204 mg), 2-fluorophenol (112 mg), ADDP (252 mg) and tri-n-butylphosphine (202 mg) was heated in toluene (5 ml) at 100° C. for 15 hours. The reaction mixture was diluted with ethyl acetate and the precipitate was removed by filtration. The filtrate was washed with aqueous NaHCO3 solution and brine, dried over Na2SO4, and concentrated in vacuo. Purification by HPLC afforded the title Compound 25 as a pale pink solid (15 mg). 1H NMR (400 MHz, DMSO-d6) δ 3.79-3.92 (m, 3H) 5.09-5.17 (m, 2H) 6.72-7.41 (m, 7H) 7.62-7.94 (m, 1H) 12.83-13.05 (m, 1H). [M+H] calc'd for C17H15FN2O2, 299; found, 299.
References:
US2007/197532,2007,A1 Location in patent:Page/Page column 48
118-93-4
548 suppliers
$10.00/25g

60943-39-7
15 suppliers
inquiry