
Ethyl 2-phenylacetoacetate synthesis
- Product Name:Ethyl 2-phenylacetoacetate
- CAS Number:5413-05-8
- Molecular formula:C12H14O3
- Molecular Weight:206.24


108-24-7
5 suppliers
$14.00/250ML
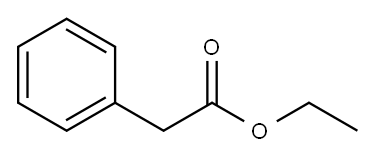
101-97-3
465 suppliers
$14.00/25mL

5413-05-8
183 suppliers
$165.00/1g
Yield:5413-05-8 61%
Reaction Conditions:
Stage #1: Ethyl 2-phenylethanoatewith sodium hydride in tetrahydrofuran at 60; for 0.5 h;Cooling with ice;
Stage #2: acetic anhydride in tetrahydrofuran at 0 - 20; for 1 h;
Steps:
1.B B Ethyl 2-phenylacetoacetate (Intermediate 2)
NaH (326 mg, 8.15 mmol) was suspended in 15 mL of dry THF.Ethyl phenylacetate (1 mL, 6.27 mmol) was added dropwise under ice bath.After the completion of the dropwise addition, the reaction was carried out at 60 ° C for 30 min.The reaction solution was cooled to 0 ° C.Add 2 mL of acetic anhydride (655 μL, 7 mmol) in THF.After the completion of the dropwise addition, the mixture was transferred to room temperature, and the reaction was continued for 1 hour.After the reaction was completed, the reaction solution was poured into ice water and extracted with EtOAc.The organic phase is washed with water, saturated with NaHCO3, and washed with saturated NaCl.Dry with anhydrous Na2SO4. concentrate,Separated by column chromatography (petroleum ether: EtOAc = 10:1).A yellow oily liquid 789 mg was obtained. Υield 61%:
References:
CN108997242,2018,A Location in patent:Paragraph 0034; 0037; 0038
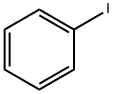
591-50-4
473 suppliers
$10.00/1g
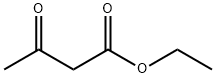
141-97-9
770 suppliers
$5.00/25g

5413-05-8
183 suppliers
$165.00/1g

101-97-3
465 suppliers
$14.00/25mL

27262-63-1
0 suppliers
inquiry

5413-05-8
183 suppliers
$165.00/1g

591-50-4
473 suppliers
$10.00/1g

141-97-9
770 suppliers
$5.00/25g

5413-05-8
183 suppliers
$165.00/1g

101-97-3
465 suppliers
$14.00/25mL

75-36-5
560 suppliers
$17.92/100G

5413-05-8
183 suppliers
$165.00/1g