
Ethyl-carbaMicacidpiperidin-4-ylester synthesis
- Product Name:Ethyl-carbaMicacidpiperidin-4-ylester
- CAS Number:70724-24-2
- Molecular formula:C8H16N2O2
- Molecular Weight:172.22
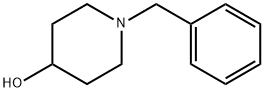
4727-72-4
325 suppliers
$29.60/5g

107-06-2
401 suppliers
$14.00/25g
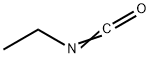
109-90-0
182 suppliers
$59.20/5g

70724-24-2
21 suppliers
$155.00/100mg
Yield:-
Reaction Conditions:
in palladium on charcoal catalyst;hexane;ethyl acetate;
Steps:
K Preparation of 4-(N-Ethylcarbamoyloxy)piperidine
PREPARATION K Preparation of 4-(N-Ethylcarbamoyloxy)piperidine 1-Benzyl-4-piperidinol (293.4 g), ethyl isocyanate (120 g) and 1,2-dichloroethane (1467 ml) were heated and stirred together under reflux for 7 hours. A further 10.9 g of ethyl isocyanate was then added and reflux was continued for another 6 hours. After cooling and standing for 36 hours at room temperature, the reaction had not proceeded to completion and so a further 33 g of ethyl isocyanate was added and heating under reflux continued for a further 6 hours. The mixture was cooled, poured into water (2000 ml) and stirred for 11/2 hours, after which the organic layer was separated, washed with saturated sodium bicarbonate solution (2000 ml) and water (2000 ml). The combined aqueous phases were further extracted with dichloroethane (150 ml) and the bulked organic phases were dried (MgSO4) and evaporated in vacuo to give a crude product which was stirred with boiling hexane, cooled, and filtered to give 1-benzyl-4-(N-ethylcarbamoyloxy)piperidine (354.4 g), m.p. 96°-98° C. This product (118 g) in industrial methylated spirit (826 ml) was hydrogenated at 50°/50 p.s.i. over 5% palladium on charcoal catalyst (12 g) until uptake of hydrogen ceased. The catalyst was then removed by filtration, the filtrate evaporated in vacuo, and the resulting residue was recrystallized from a mixture of hexane (450 ml) and ethyl acetate (112 ml) to give 4-(N-ethylcarbamoyloxy)piperidine (208.1 g), m.p. 85°-87°, used directly in Example 12.
References:
US4243666,1981,A