GSK J4 HCl synthesis
- Product Name:GSK J4 HCl
- CAS Number:1373423-53-0
- Molecular formula:C24H27N5O2
- Molecular Weight:417.5
![2,3,4,5-TETRAHYDRO-1H-BENZO[D]AZEPINE](/CAS/GIF/4424-20-8.gif)
4424-20-8
130 suppliers
$49.00/100mg

1373423-17-6
8 suppliers
inquiry

1373423-53-0
103 suppliers
$110.00/10mg
Yield:-
Reaction Conditions:
in dimethyl sulfoxide;
Steps:
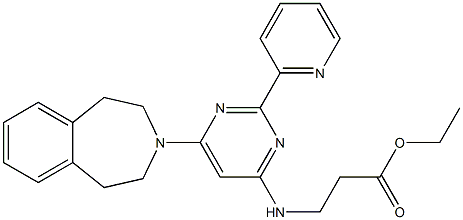
Ethyl N-[2-(2-pyridinyl)-6-(1,2,4,5-tetrahydro-3H-3-benzazepin-3-yl)-4-pyrimidinyl]-β-alaninate
Ethyl N-[2-(2-pyridinyl)-6-(1,2,4,5-tetrahydro-3H-3-benzazepin-3-yl)-4-pyrimidinyl]-β-alaninate
To a microwave vial with a stirrer was added 2,3,4,5-tetrahydro-1H-3-benzazepine (0.049 mL, 0.326 mmol), ethyl N-[6-chloro-2-(2-pyridinyl)-4-pyrimidinyl]-β-alaninate (100 mg, 0.326 mmol), DIPEA (0.114 mL, 0.652 mmol) and Dimethyl Sulfoxide (DMSO) (0.5 mL).
The reaction vessel was sealed and heated in a Biotage Initiator microwave to 150 °C for 2.5 hr.
After allowing the reaction mixture to cool, analysis by LCMS showed 73% conversion to the product.
The reaction mixture was dissolved in DMSO to make the sample up to 1 mL and purified by MDAP. Product containing fractions were combined and the solvent evaporated in vacuo to give the title compound (99 mg) as a yellow oil.
References:
EP2592154,2013,A1 Location in patent:Page/Page column

10235-65-1
81 suppliers
$42.00/100mg

1373423-53-0
103 suppliers
$110.00/10mg
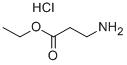
4244-84-2
325 suppliers
$5.00/1g

1373423-53-0
103 suppliers
$110.00/10mg