
1,2,4-cyclohexanetricarboxylic anhydride synthesis
- Product Name:1,2,4-cyclohexanetricarboxylic anhydride
- CAS Number:53611-01-1
- Molecular formula:C9H10O5
- Molecular Weight:198.17
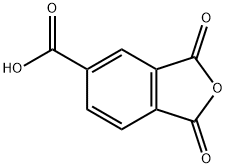
552-30-7
391 suppliers
$16.00/25g

53611-01-1
89 suppliers
inquiry
Yield:53611-01-1 98.9%
Reaction Conditions:
with hydrogen;palladium in acetone at 100; under 28127.8 Torr;Autoclave;Temperature;Pressure;Reagent/catalyst;Solvent;
Steps:
B8 Example 1
General procedure: The mixture of 60g of trimellitic anhydride, 120g of anhydrous acetone and 3g of palladium catalyst was added to the high-pressure reactor, the reaction temperature of the autoclave was maintained at 100 °C, the pressure of the hydrogenation reaction was maintained at 2.0Mpa, the reaction solution was taken every 2h for the catalytic hydrogenation reaction, tested with liquid chromatography, and the content of trimellitic anhydride was detected to be less than 1%, and the content of 1,2,4-cyclohexanetricartic anhydride was higher than 98%, the hydrogenation reaction was stopped, and after filtering the catalyst, the liquid phase was collected, and part of the solvent was distilled. Finally, 1,2,4-cyclohexane tricarboxylic anhydride solids were obtained. 1,2,4-Cyclohexanetricarboxylic anhydride yield was tested at 95.0%.
References:
CN113831310,2021,A Location in patent:Paragraph 0017-0023

23084-86-8
3 suppliers
inquiry

53611-01-1
89 suppliers
inquiry