Imiquimod synthesis
- Product Name:Imiquimod
- CAS Number:99011-02-6
- Molecular formula:C14H16N4
- Molecular Weight:240.3
![4-Chloro-1-(2-methylpropyl)-1H-imidazo[4,5-c]quinoline](/CAS/20180906/GIF/99010-64-7.gif)
99010-64-7
158 suppliers
$49.00/10g

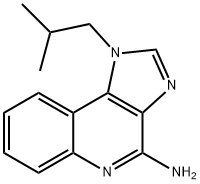
99011-02-6
485 suppliers
$6.00/100mg
Yield:99011-02-6 85.7%
Reaction Conditions:
with ammonia in dimethyl sulfoxide at 20 - 150; under 1875.19 - 3750.38 Torr; for 6 - 12 h;Product distribution / selectivity;
Steps:
1; 2
This example demonstrates the preparation of imiquimod by reaction of compound II with ammonia in DMSO at 140-150° C. under a pressure of about 5 bar.A 250 glass reactor “Miniclave” was charged with 4-chloro-1-isobutyl-1H-imidazo [4,5-c]quinoline (II) (20 g, 0.0733 mol) and DMSO (50 ml). Ammonia gas (2 g, 0.118 mol, 1.6 equiv.) was added into the closed reactor and the mixture was heated under stirring to 145-150° C. to obtain a pressure sustaining a glass reactor of about 5 bars. After heating at 140-150° C. for 10 hours the pressure was reduced to atmospheric pressure and the reaction mixture was cooled to ambient temperature. A sample was withdrawn and injected to an HPLC system. According to the HPLC chromatogram the product contained 51 % of imiquimod and 49% of the compound II in the reaction mixture.Then, a second portion of ammonia gas (2 g) was added into the closed reactor at ambient temperature followed by heating the reaction mixture to 145° C. to obtain a pressure of about 5 bar. The heating was continued for 12 hours during which time the pressure was reduced to 2.5 bar. The reaction mixture was cooled to ambient temperature and a sample was withdrawn and injected to an HPLC system. According to the HPLC chromatogram the product contained 99.93% of imiquimod and 0.07% of compound II in the reaction mixture; This example demonstrates the preparation of imiquimod by reaction of compound II with ammonia in DMSO at 140-150° C. under a pressure of about 5 bar.A 250 glass reactor “Miniclave” was charged with 4-chloro-1-isobutyl-1H-imidazo [4,5-c]quinoline (II) (20 g, 0.0733 mol) and DMSO (50 ml). Ammonia gas (2 g, 0.118 mol, 1.6 equiv.) was added into the closed reactor and the mixture was heated under stirring to 145-150° C. to obtain a pressure sustaining a glass reactor of about 5 bars. After heating at 140-150° C. for 10 hours the pressure was reduced to atmospheric pressure and the reaction mixture was cooled to ambient temperature.Then, a second portion of ammonia gas (2 g) was added into the closed reactor at ambient temperature followed by heating the reaction mixture to 145° C. to obtain a pressure of about 5 bar. The heating was continued for 6 hours during which time the pressure was reduced to 2.5 bar. The reaction mixture was cooled to ambient temperature and a sample was withdrawn and injected to an HPLC system. According to the HPLC chromatogram the product contained about 92% of imiquimod and 8% of compound II in the reaction mixture.A colorless precipitate was collected by filtration and washed with water (3×50 ml). The wet product was treated with water (80 ml) under stirring at 70-80° C. for 1 hour. Then, the hot suspension was filtered and the precipitate was washed with hot water (40 ml) and methanol (40 ml) and dried at 80° C. under reduced pressure to yield 16.0 g of crude imiquimod in 85.7% yield; having a purity of 99.9% (by HPLC).
References:
CHEMAGIS LTD. US2008/194822, 2008, A1 Location in patent:Page/Page column 2-3
![Imiquimod Related Compound B (25 mg) (1-Isobutyl-1H-imidazo[4,5-c]quinoline 5-oxide)](/CAS/20150408/GIF/99010-63-6.gif)
99010-63-6
43 suppliers
inquiry

99011-02-6
485 suppliers
$6.00/100mg

960254-02-8
1 suppliers
inquiry

99011-02-6
485 suppliers
$6.00/100mg

896106-15-3
1 suppliers
inquiry

99011-02-6
485 suppliers
$6.00/100mg
![4-chloro-3H-imidazo[4,5-C]quinoline](/CAS/20180808/GIF/132206-92-9.gif)
132206-92-9
15 suppliers
inquiry

99011-02-6
485 suppliers
$6.00/100mg