Isobutylbenzene synthesis
- Product Name:Isobutylbenzene
- CAS Number:538-93-2
- Molecular formula:C10H14
- Molecular Weight:134.22


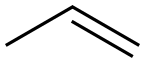
5674-02-2
75 suppliers
$66.29/100ml
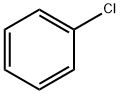
108-90-7
643 suppliers
$10.00/25G

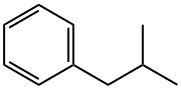
538-93-2
205 suppliers
$14.00/5mL
Yield:538-93-2 92%
Reaction Conditions:
with iron(II) chloride tetrahydrate;1,3-bis[2,6-diisopropylphenyl]imidazolium chloride in tetrahydrofuran at 70; for 3 h;Inert atmosphere;
Steps:
Representative cross-coupling procedure
General procedure: 1,3-Bis-(2,6-diisopropylphenyl)imidazolium chloride (0.1 mmol, 10 mol %) was added to Vial 1 containing a stir bar which was then fitted with a septum. FeCl2·(H2O)4 (9.9 mg, 0.05 mmol, 5 mol %) was added to Vial 2 containing a stir bar which was then fitted with a septum. Both Vial 1 and Vial 2 were evacuated and backfilled with argon. Chlorobenzene (102 μL, 1 mmol) was then added via syringe to Vial 2. Freshly distilled THF (10.5 mL) was added via syringe to Vial 1, and isobutylmagnesium chloride (1.5 mL of a 2 M solution in THF, 3 mmol) was added with stirring. Vial 1 was placed in an oil bath at 70 °C and stirred for 10 min. Then the contents of Vial 1 were transferred to Vial 2 via syringe, and Vial 2 was placed in the oil bath at 70 °C. After 3 h, the reaction was removed from the oil bath and allowed to cool to room temperature. The reaction mixture was poured into a separatory funnel followed by 15 mL of 1 M HCl and 15 mL of pentane. The pentane layer was washed with water (1 × 15 mL) and brine (1 × 15 mL). The pentane layer was dried over Na2SO4 and the solvent was removed in vacuo. The crude oil was purified by distillation resulting in a colorless oil (122 mg, 92%). 1H NMR and GC-MS were consistent with known material.
References:
Perry, Marc C.;Gillett, Amber N.;Law, Tyler C. [Tetrahedron Letters,2012,vol. 53,# 33,p. 4436 - 4439] Location in patent:experimental part
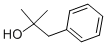
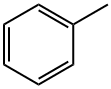
100-86-7
301 suppliers
$8.00/25g

538-93-2
205 suppliers
$14.00/5mL
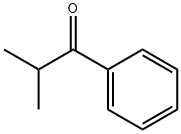
611-70-1
173 suppliers
$6.00/5g

538-93-2
205 suppliers
$14.00/5mL

768-49-0
109 suppliers
$18.00/100mg

538-93-2
205 suppliers
$14.00/5mL