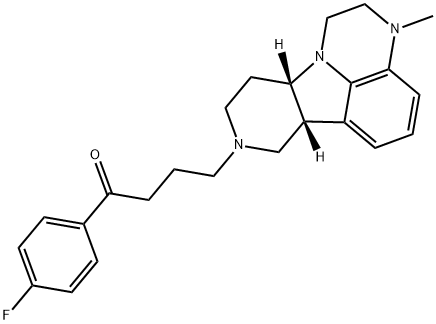
ITI-722 synthesis
- Product Name:ITI-722
- CAS Number:313368-91-1
- Molecular formula:C24H28FN3O
- Molecular Weight:393.5

Li, Peng; Zhang, Qiang; Robichaud, Albert J.; Lee, Taekyu; Tomesch, John; Yao, Wei; Beard, J. David; Snyder, Gretchen L.; Zhu, Hongwen; Peng, Youyi; Hendrick, Joseph P.; Vanover, Kimberly E.; Davis, Robert E.; Mates, Sharon; Wennogle, Lawrence P. Discovery of a Tetracyclic Quinoxaline Derivative as a Potent and Orally Active Multifunctional Drug Candidate for the Treatment of Neuropsychiatric and Neurological Disorders. Journal of Medicinal Chemistry. Intra-Cellular Therapies, Inc. New York, USA 10032Volume 57. Issue 6. Pages 2670-2682. 2014.
Yield:313368-91-1 84%
Reaction Conditions:
with caesium carbonate in acetonitrile at 20; for 24 h;Reagent/catalyst;Solvent;Temperature;
Steps:
9; 10 Method B
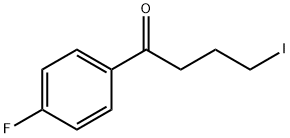
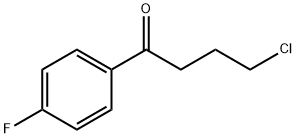
0.459 g (2.0 mmol) formula (II) compound is dissolved in 15 ml acetonitrile, then, to this, 1.303 g (4.0 mmol) caesium carbonate and 0.876 g (3.0 mmol) 4-iodo-4'-fluoro butyrophenone is added. The reaction mixture is stirred for 24 hours at room temperature. Then it is filtered and evaporated. The evaporation residue is dissolved in 20 ml dichlorom ethane and then extracted with 10 ml water, than with 10 ml saturated NaCl solution. The organic phase is dried on Na2S04, filtered and evaporated. The 1.06 g raw product obtained is cleaned on silica gel with flash chromatography using CfTCb-MeOH as eluent. 0.66 g (84%) formula (I) compound is obtained (light brown oil).
References:
WO2019/102240,2019,A1 Location in patent:Page/Page column 75; 76
![1H-Pyrido[3',4':4,5]pyrrolo[1,2,3-de]quinoxaline, 2,3,6b,7,8,9,10,10a-octahydro-3-methyl-](/CAS/20200611/GIF/313369-67-4.gif)
313369-67-4
0 suppliers
inquiry

313368-91-1
74 suppliers
inquiry


![(6bR,10aS)-3-Methyl-2,3,6b,7,8,9,10,10a-octahydro-1H-pyrido[3',4':4,5]pyrrolo[1,2,3-de]quinoxaline](/CAS/20180527/GIF/313368-85-3.gif)