JAK3-IN-6 synthesis
- Product Name:JAK3-IN-6
- CAS Number:1443235-95-7
- Molecular formula:C19H18N4O3
- Molecular Weight:350.37
Yield:-
Reaction Conditions:
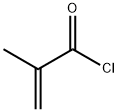
with N-ethyl-N,N-diisopropylamine in tetrahydrofuran at 15 - 25; for 1 h;Inert atmosphere;
Steps:
4-1 Ethyl-4- ( 3 - [(2-methylacryloyl)amino]phenyl } -7H-pyrrolo [2,3 -ci]pyrimidine-5- carboxylate (4-1)
To a solution of ethyl 4-(3-aminophenyl)-7H-pyrrolo[2,3-iflpyrimidine-5- carboxylate (50 mg, 0.18 mmol) and DIPEA (93 μ,, 0.53 mmol) in THF (1.8 mL) at room temperature was added methacryloyl chloride (27.8 mg, 0.266 mmol) and the solution stirred for 1 hour. The reaction was quenched with ethanol (0.1 mL). The desired product was purified by reverse phase HPLC using an acetonitrile gradient in water with 0.1% TFA modifier. The combined fractions with the desired product were concentrated until only the water layer remained. The water layer was neutralized with 2M aqueous potassium bicarbonate and the mixture was extracted with ethyl acetate (x5). The combined organic fractions were dried over sodium sulfate, filtered and concentrated under reduced pressure to give ethyl 4-{3-[(2-methylacryloyl)amino]phenyl}-7H- pyrrolo[2,3-^pyrimidine-5-carboxylate. LRMS (ESI) calc'd for Ci9H19N403 [M+H]+: 351, found 351. NMR (600 MHz, DMSO-D6) δ 13.0 (s, 1H), 9.91 (s, 1H), 8.91 (s, 1H), 8.30 (s, 1H), 8.05 (s, 1H), 7.84 (d, 1H, J = 7.04 Hz), 7.40 (t, 1H, J= 7.63 Hz), 7.32 (d, 1H, J = 7.04 Hz), 5.82 (s, 1H), 5.53 (s, 1H), 3.86 (q, 2H, J = 7.04 Hz), 1.95 (s, 3H), 0.85 (t, 3H, J= 7.04 Hz).
References:
WO2013/85802,2013,A1 Location in patent:Page/Page column 82
![5-Bromo-4-chloro-7H-pyrrolo[2,3-d]pyrimidine](/CAS/GIF/22276-95-5.gif)
22276-95-5
200 suppliers
$8.00/1g

1443235-95-7
51 suppliers
inquiry
![ethyl 4-chloro-7H-pyrrolo[2,3-d]pyrimidine-5-carboxylate](/CAS/20180808/GIF/144927-57-1.gif)
144927-57-1
100 suppliers
$62.00/100mg

1443235-95-7
51 suppliers
inquiry
![4-Chloro-7H-pyrrolo[2,3-d]pyrimidine](/CAS/GIF/3680-69-1.gif)
3680-69-1
846 suppliers
$6.00/1g

1443235-95-7
51 suppliers
inquiry
![7H-Pyrrolo[2,3-d]pyrimidine-5-carboxylic acid, 4-(3-aminophenyl)-, ethyl ester](/CAS/20210305/GIF/1443234-84-1.gif)