L-Carnosine synthesis
- Product Name:L-Carnosine
- CAS Number:305-84-0
- Molecular formula:C9H14N4O3
- Molecular Weight:226.23
Yield:-
Reaction Conditions:
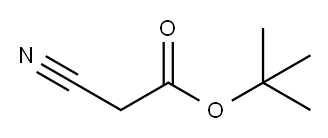
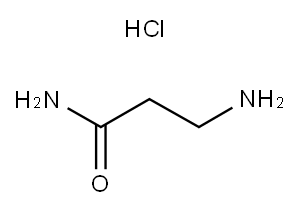
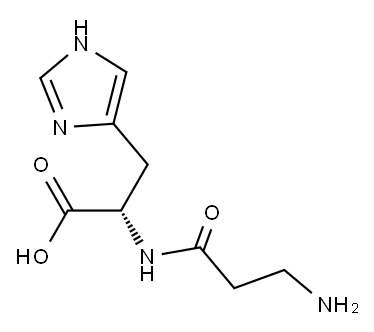
Stage #1: cyanoacetic acid tert-butyl ester;L-histidinewith 4-chloropyrimidine;pyridine-4-carboxylic acid;sodium methoxide in N,N-dimethyl-formamide at 95; for 4.5 h;
Stage #2: with hydrogenchloride in water monomer;N,N-dimethyl-formamide at 0; for 8 h;
Stage #3: with ammonium hydroxide;rhodium on charcoal;hydrogen in ethanol at 62; under 150015 Torr; for 24 h;Pressure;Temperature;Reagent/catalyst;
Steps:
1.1; 1.2 1. Synthesis of carnosine intermediates
Take 30 g of L-histidine, 34 g of tert-butyl cyanoacetate, 3 g of sodium ethoxide, 1.2 g of isonicotinic acid, 1.5 g of 4-chloropyrimidine, and 150 g of DMF, mix well, heat up to 95 °C and react for 4.5 h, then reduce Continue to add 25 g of hydrochloric acid, cool to 0 °C and stir for 8 h, then stand for crystallization and suction filtration; dissolve the obtained crystals in distilled water, adjust the pH to 4.2 with hydrochloric acid, recrystallize and filter with suction to obtain the carnosine intermediate2. Carnosine SynthesisTake 25 g of carnosine intermediate, 1.2 g of rhodium carbon, 30 g of ammonia water,120 g of ethanol was mixed and added to the reaction vessel; after vacuuming, nitrogen was introduced to replace the air, and then hydrogen was introduced to the pressure of 20 MPa; after 62 °C reaction for 24 h, the temperature was cooled to room temperature, and the rhodium carbon was removed by activated carbon, and filtered; the filtrate was concentrated After drying, add 200 g of ethanol, extract under heat reflux for 15 min, filter and dry to obtain carnosine.
References:
CN114213335,2022,A Location in patent:Paragraph 0039; 0040
111196-17-9
26 suppliers
$60.00/500mg
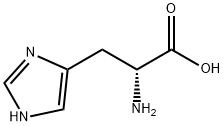
351-50-8
349 suppliers
$10.00/1g

305-84-0
656 suppliers
$10.00/1g