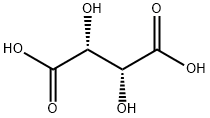
L(+)-Tartaric acid synthesis
- Product Name:L(+)-Tartaric acid
- CAS Number:87-69-4
- Molecular formula:C4H6O6
- Molecular Weight:150.09
Commercially, L-(+)-tartaric acid is manufactured from potassium tartrate (cream of tartar), a by-product of wine making. Potassium tartrate is treated with hydrochloric acid, followed by the addition of a calcium salt to produce insoluble calcium tartrate. This precipitate is then removed by filtration and reacted with 70% sulfuric acid to yield tartaric acid and calcium sulfate.

7760-07-8
0 suppliers
inquiry

33012-62-3
10 suppliers
inquiry

7558-19-2
0 suppliers
inquiry

13171-74-9
2 suppliers
inquiry

87-69-4
891 suppliers
$5.00/25g
Yield:-
Reaction Conditions:
with oxygen in water at 110; under 3750.38 Torr;Autoclave;Pressure;Temperature;
References:
Ding, Jie;Jin, Xin;Lai, Linyi;Liu, Mengyuan;Sun, Yu;Wang, Jinyao;Xia, Qi;Yan, Hao;Yang, Chaohe;Zhang, Guangyu;Zhang, Wenxiang [ACS Catalysis,2020,vol. 10,# 19,p. 10932 - 10945]
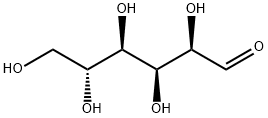
50-99-7
978 suppliers
$6.00/25g
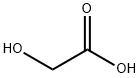
79-14-1
754 suppliers
$6.00/25g

5627-26-9
1 suppliers
inquiry

87-69-4
891 suppliers
$5.00/25g

50-99-7
978 suppliers
$6.00/25g

79-14-1
754 suppliers
$6.00/25g
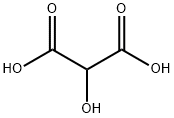
80-69-3
91 suppliers
$7.00/100mg

5627-26-9
1 suppliers
inquiry

87-69-4
891 suppliers
$5.00/25g

50-99-7
978 suppliers
$6.00/25g

79-14-1
754 suppliers
$6.00/25g

80-69-3
91 suppliers
$7.00/100mg
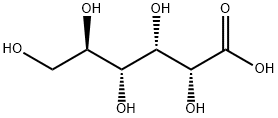
526-95-4
381 suppliers
$5.00/5g

87-69-4
891 suppliers
$5.00/25g

526-95-4
381 suppliers
$5.00/5g

79-14-1
754 suppliers
$6.00/25g

80-69-3
91 suppliers
$7.00/100mg

5627-26-9
1 suppliers
inquiry

87-69-4
891 suppliers
$5.00/25g