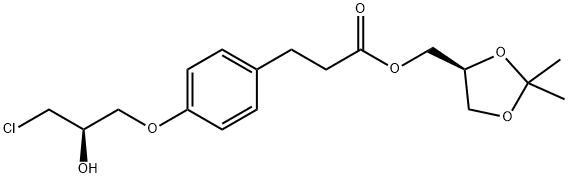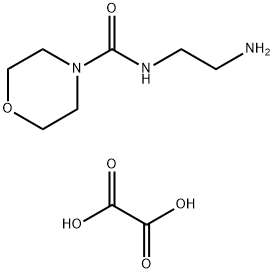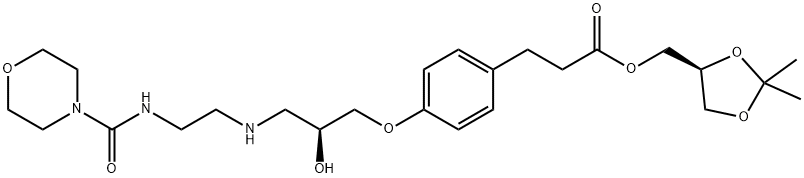
Landiolol synthesis
- Product Name:Landiolol
- CAS Number:133242-30-5
- Molecular formula:C25H39N3O8
- Molecular Weight:509.59
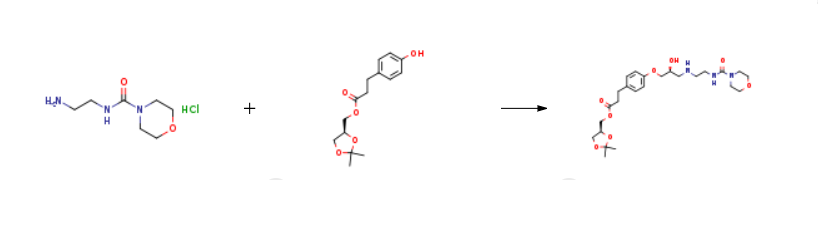
A suspension of (S)-(2,2-dimethyl-1,3-dioxolan-4-yl)methyl 3-(4-((2R)-3-chloro-2-hydroxypropoxy)phenyl)propanoate prepared according Example 3 (0.50 g, 0.00134 mol) in isopropanol (10 ml) is added with 2-(morpholine-4-carboxamido)ethanamino hydrochloride (18) (1.4 g, 0.00670 mol), heated to 30-35°C and dropwise added with 30% NaOH, keeping pH at 10-11. The mixture is left under stirring at 35-40°C, monitoring by UPLC. After completion of the reaction, ethyl acetate (20 ml) and water (20 ml) are added and the phases are separated. The organic phase is added with water (20 ml) and adjusted to pH 3-4 with hydrochloric acid. The phases are separated and the resulting aqueous phase is then adjusted to pH 10-11 with sodium hydroxide and re-extracted with ethyl acetate (20 ml). The solvent is then evaporated off under reduced pressure to obtain 0.38 g (55.6%) of a pale yellow oil which solidifies in time to a pale yellow solid.
Yield:133242-30-5 70.7%
Reaction Conditions:
Stage #1: (S)-(2,2-dimethyl-1,3-dioxolan-4-yl)methyl 3-(4-((2R)-3-chloro-2-hydroxypropoxy)phenyl)propanoatewith potassium carbonate;potassium iodide in acetonitrile; for 2 h;Reflux;
Stage #2: N-(2-aminoethyl)-4-morpholine carboxamide oxalic acid in acetonitrile;Reflux;
Steps:
5 Example 5
Landiolol (1)
Example 5
Landiolol (1)
A solution of (S)-(2,2-dimethyl-1,3-dioxolan-4-yl)methyl 3-(4-((2R)-3-chloro-2-hydroxypropoxy)phenyl)propanoate (5) prepared according to Example 3 (0.30 g, 0.805 mmol) in acetonitrile (6.0 ml) is added with potassium carbonate 0.45 g (3.22 mmol), and KI 0.013 g (0.0805 mmol), then refluxed for 2 h and added with 2-(morpholine-4-carboxamido)ethanamino oxalate (6) (0.64 g, 2.42 mmol).
The mixture is refluxed under stirring, monitoring by UPLC.
After completion of the reaction, ethyl acetate (10 ml) and water (10 ml) are added and the phases are separated.
The organic phase is added with water (10 ml) and adjusted to pH 4-5 with hydrochloric acid, the phases are separated and the resulting aqueous phase is then adjusted to pH 11-12 with sodium hydroxide and re-extracted with ethyl acetate (10 ml).
Then the solvent is evaporated off under reduced pressure to obtain 0.29 g (70.7%) of a pale yellow oil which solidifies in time to a pale yellow solid.
LC-MS (ESI+) [M+H]+ = 510
1H-NMR (CDCl3) (chemical shifts expressed in ppm with respect to TMS) (assigned based on the hetero correlation HSQC spectrum): 1.36 (3H, s, CH3); 1.42 (3H, s, CH3); 2.63 (2H, t, J = 7 Hz, CH2-Ar); 2.75 - 2.93 (8H, m, CH2-CO, CH-CH2-NH, CH2-CH2-NH, NH and OH); 3.35 (6H, m, 2CH2-N morpholine and CH2-NH); 3.65 (4H, m, 2CH2-O morpholine), 3.68 (1H, m, CH in 4 oxolane); 3.94 (2H, bd, CH2-OAr); 4.00 - 4.20 (4H, m, CH in 4 oxolane, CH2-OCO and CH in 5 oxolane); 4.25 (1H, m, CH-OH); 5.21 (1H, bt, NH carbamate); 6.83 and 7.11 (4H, system AA'XX', aromatics).
13C-NMR (CDCl3) (ppm) (multiplicity was assigned by DEPT-135): 25.3 (CH3); 26.6 (CH3); 29.9 (CH2); 35.8 (CH2); 40.2 (CH2); 43.8 (CH2-N morpholine); 49.2 (CH2); 51.5 (CH2); 64.6 (CH2); 66.2 (CH2); 66.4 (CH2-O morpholine); 68.3 (CH); 70.3 (CH2); 73.4 (CH); 109.7; 114.4 (CH); 129.2 (CH); 132.8; 157.0; 158.0; 172.5 (COOR).
FT-IR (UATR, cm-1): 3350. 2858, 1735, 1626, 1512, 1454, 1371, 1244, 1153, 1115, 1040. 829, 733.
References:
EP2687521,2014,A1 Location in patent:Paragraph 0045; 0046; 0047; 0048; 0049; 0050

144256-11-1
87 suppliers
inquiry

133242-30-5
62 suppliers
inquiry

93605-74-4
13 suppliers
inquiry

133242-30-5
62 suppliers
inquiry
501-97-3
514 suppliers
$10.00/5g

133242-30-5
62 suppliers
inquiry