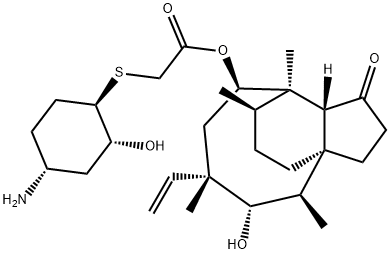
Lefamulin synthesis
- Product Name:Lefamulin
- CAS Number:1061337-51-6
- Molecular formula:C28H45NO5S
- Molecular Weight:507.73

Reference: Heilmayer, Werner; Mang, Rosemarie; Badegruber, Rudolf; Stickmann, Dirk; Novak, Rodger; Ferencic, Mathias; Bulusu, Atchyuta Rama Chandra Murty. Synthesis of pleuromutilin derivatives for the treatment of diseases mediated by microbes. MX 2010009451. (2012).
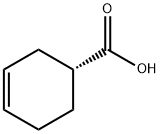
5709-98-8
180 suppliers
$13.00/1g

1061337-51-6
49 suppliers
inquiry
Yield:-
Steps:
Multi-step reaction with 7 steps
1.1: triethylamine; diphenyl phosphoryl azide / toluene / 0.58 h / 20 - 95 °C / Reflux
2.1: copper(l) chloride / 0.83 h / 80 - 100 °C
3.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 3.5 h / 15 - 40 °C / Industry scale
4.1: tetrabutyl-ammonium chloride / toluene / 4 h / 30 - 45 °C / Industry scale
5.1: hydrazine hydrate; DL-dithiothreitol / dichloromethane / 3 h / 18 - 22 °C / Industry scale
6.1: sodium hydroxide / benzyltri(n-butyl)ammonium chloride / water; tert-butyl methyl ether / 3 h / 17 - 23 °C / Industry scale
7.1: phosphoric acid / isopropyl alcohol / 16 h / 50 °C / Industry scale
7.2: 1.5 h / 0 - 25 °C / Industry scale
References:
NABRIVA THERAPEUTICS AG;RIEDL, Rosemarie;HEILMAYER, Werner;SPENCE, Lee WO2011/146954, 2011, A1
![Acetic acid, 2-[[(1R,2R,4R)-2-hydroxy-4-[(2,2,2-trifluoroacetyl)amino]cyclohexyl]thio]-, (3aS,4R,5S,6S,8R,9R,9aR,10R)-6-ethenyldecahydro-5-hydroxy-4,6,9,10-tetramethyl-1-oxo-3a,9-propano-3aH-cyclopentacycloocten-8-yl ester](/CAS/20210111/GIF/1350636-79-1.gif)
1350636-79-1
0 suppliers
inquiry

1061337-51-6
49 suppliers
inquiry
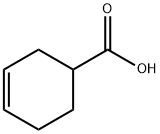
4771-80-6
264 suppliers
$5.00/10g

1061337-51-6
49 suppliers
inquiry

1350636-88-2
4 suppliers
inquiry

1061337-51-6
49 suppliers
inquiry


![Acetic acid, 2-[[4-[[(1,1-dimethylethoxy)carbonyl]amino]-2-hydroxycyclohexyl]thio]-, (3aS,4R,5S,6S,8R,9R,9aR,10R)-6-ethenyldecahydro-5-hydroxy-4,6,9,10-tetramethyl-1-oxo-3a,9-propano-3aH-cyclopentacycloocten-8-yl ester](/CAS/20210305/GIF/1061660-17-0.gif)
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)