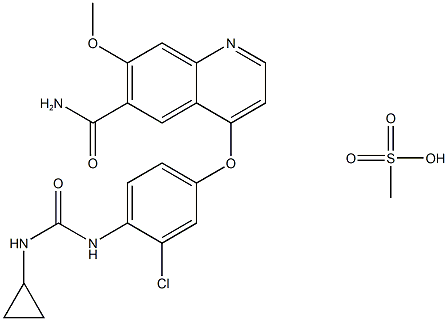
lenvatinib Mesylate synthesis
- Product Name:lenvatinib Mesylate
- CAS Number:857890-39-2
- Molecular formula:C22H23ClN4O7S
- Molecular Weight:522.9586
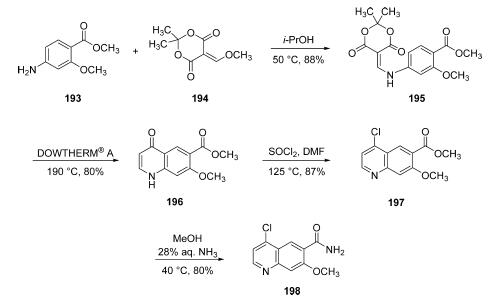
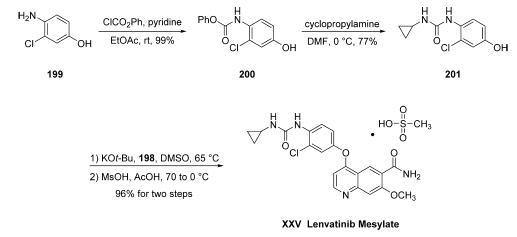
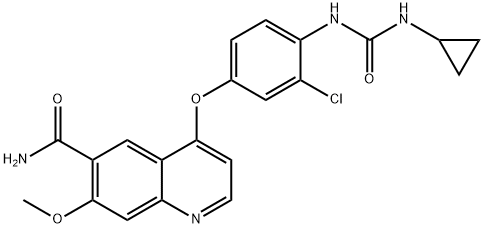
Commercial aminophenol 199 was converted to the corresponding carbamate through the use of phenyl chloroformate in essentially quantitative yield prior to subjection to cyclopropylamine in chilled DMF, which ultimately furnished urea 201 in 77% overall yield from 200. Next, exposure of phenol 201 to chloroquinoline 198 in the presence of potassium t-butoxide followed by treatment with methanesulfonic acid and acetic acid resulted in clean formation of lenvatinib mesylate (XXV) in 96% yield across the two-step sequence.
417716-92-8
458 suppliers
$5.00/10mg
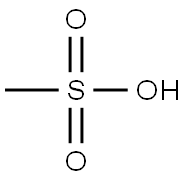
75-75-2
654 suppliers
$10.00/5g

857890-39-2
392 suppliers
$29.00/5mg
Yield:857890-39-2 99%
Reaction Conditions:
in tetrahydrofuran;lithium hydroxide monohydrate at 20; for 3.66667 h;
Steps:
1 Preparation of compound 5.
Weigh compound 4 (0.33 g, 0.77 mmol) into a 50 mL eggplant-shaped flask. Add 7 mL of THF and 3 mL of water, and stir well. Prepare a mixed solution of methanesulfonic acid and water (0.4mL methanesulfonic acid, 1.6mL water), add 0.5mL of the prepared methanesulfonic acid aqueous solution dropwise to the eggplant-shaped flask, stir at room temperature for ten minutes, continue to add 0.4 mL methanesulfonic acid aqueous solution, after stirring for 30 min, continue to add 0.1 mL of methanesulfonic acid aqueous solution, and after stirring for 2 hours at room temperature, after adding 1 mL of methanesulfonic acid aqueous solution and reacting for 1 hour, after suction filtration, it was dried in a blast drying cabinet to obtain compound 5: 0.40 g, with a yield of 99%.
References:
CN111349045,2020,A Location in patent:Paragraph 0049; 0062-0064

417722-95-3
48 suppliers
inquiry

75-75-2
654 suppliers
$10.00/5g
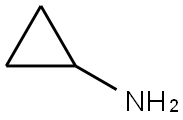
765-30-0
507 suppliers
$10.00/25g

857890-39-2
392 suppliers
$29.00/5mg
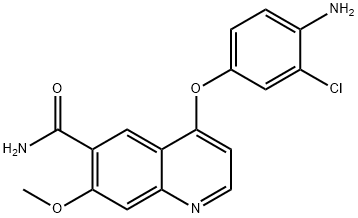
417722-93-1
101 suppliers
inquiry

857890-39-2
392 suppliers
$29.00/5mg
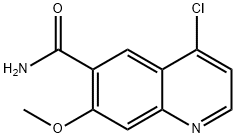
417721-36-9
357 suppliers
$8.00/250mg

857890-39-2
392 suppliers
$29.00/5mg

52671-64-4
331 suppliers
$7.00/1g

857890-39-2
392 suppliers
$29.00/5mg