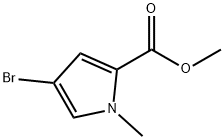
METHYL 4-BROMO-1-METHYL-1H-PYRROLE-2-CARBOXYLATE synthesis
- Product Name:METHYL 4-BROMO-1-METHYL-1H-PYRROLE-2-CARBOXYLATE
- CAS Number:1196-90-3
- Molecular formula:C7H8BrNO2
- Molecular Weight:218.05
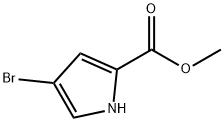
934-05-4
190 suppliers
$8.00/1g

74-88-4
341 suppliers
$15.00/10g

1196-90-3
87 suppliers
$22.00/100mg
Yield: 986 mg
Reaction Conditions:
Stage #1:methyl 4-bromo-1H-pyrrole-2-carboxylate with sodium hydride in N,N-dimethyl-formamide at 0; for 0.5 h;Inert atmosphere;
Stage #2:methyl iodide in N,N-dimethyl-formamide at 20; for 18 h;
Steps:
2.1 Example 2: Preparation of l-methyl-4-pyridin-4-yl-lH-pyrrole-2-carboxylic acid amide Step 1: Preparation of 4-bromo-l-methyl-lH-pyrrole-2-carboxylic acid methyl ester
NaH (256 mg, 6.40 mmol) was added into DMF (5 mL) and stirred at 0 °C under nitrogen for 10 min. A solution of 4-bromo-lH-pyrrole-2-carboxylic acid methyl ester (1 g, 4.93 mmol) in DMF (5 mL) was added in 15 min at 0 °C. The resulting mixture was stirred at 0°C for 30 min. Mel (909 mg, 6.40 mmol) was added to the reaction mixture. After being stirred for another 18 h at room temperature, the mixture was poured into water, and then extracted with MTBE twice. The combined organic layers were washed with brine, and then dried over anhydrous Na2S04, then filtered and concentrated in vacuo. The residue was purified by column chromatography to afford 4-bromo-l -methyl- lH-pyrrole-2-carboxylic acid methyl ester (986 mg) as a solid.
References:
F. HOFFMANN-LA ROCHE AG;HOFFMANN-LA ROCHE INC.;HAN, Xingchun;JIANG, Min;MAYWEG, Alexander V.;WANG, Lisha;YANG, Song WO2014/154723, 2014, A1 Location in patent:Page/Page column 31-32

124-41-4
681 suppliers
$12.00/25g

184643-69-4
22 suppliers
$118.00/1 g

1196-90-3
87 suppliers
$22.00/100mg

67-56-1
733 suppliers
$7.29/5ml-f

184643-69-4
22 suppliers
$118.00/1 g

1196-90-3
87 suppliers
$22.00/100mg
96-54-8
264 suppliers
$5.00/1g

1196-90-3
87 suppliers
$22.00/100mg
21898-65-7
72 suppliers
$68.00/1 g

1196-90-3
87 suppliers
$22.00/100mg