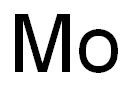
Molybdenum synthesis
- Product Name:Molybdenum
- CAS Number:7439-98-7
- Molecular formula:Mo
- Molecular Weight:95.94
10241-05-1
123 suppliers
$34.00/5g
7439-98-7
301 suppliers
$17.00/1g
Yield:-
Reaction Conditions:
with ammonia in neat (no solvent) at 899.84; for 12 h;Temperature;
Steps:
General procedure: The materials used as reagents in this investigation were NbCl5 (Aldrich, 99 %) and MoCl5 (Merck, 98 %), which were dissolved in ethanol (CH3CH2OH, Panreac) to prepare the starting Nb or Mo-containing solutions. The stoichiometric solutions were combined to obtain Nb-Mo source solutions, with nominal molar compositions Nb:Mo = 1 - x:x (x = 0.0, 0.2, 0.4, 0.5, 0.6, 0.8, 1.0). The resulting solutions were evaporated to precipitate the precursors. Niobium-molybdenum nitrides were synthesized by ammonolysis of the appropriate amorphous or crystalline precursors. The gases used in the ammonolysis were NH3 (99.9 %) and N2 (99.9995 %). A sample of the selected precursor (ca. 0.5 g) was placed in an alumina crucible which was then inserted placed in a quartz flow-through tube furnace. The gas input of the tube furnace was connected to the gas line and the gas output was connected to an acetic acid trap. Before initiation of the thermal treatment, the tube furnace was purged for 15 min with N2 and another 15 min with NH3. Several runs under different experimental conditions were also performed to determine the appropriate conditions for preparation of pure samples. The precursor powder was heated at 5 K min-1 to a final temperature (Tf) that was held for a period of time (thold) under flowing ammonia (50 cm3 min-1). The solid was then cooled at different rates (rc) in the same atmosphere, either by turning off the oven and leaving the sample inside (slow cooling, ca. 4 K min-1) or byquenching at room temperature (fast cooling, ca 50 K min-1). After cooling, the products were always passivated with flowing nitrogen for 15 min.
References:
El Himri, Abdelouahad;El Himri, Mamoune;Pérez-Coll, Domingo;Núñez, Pedro [Research on Chemical Intermediates,2015,vol. 41,# 9,art. no. 1749,p. 6397 - 6407]

10241-05-1
123 suppliers
$34.00/5g
7439-98-7
301 suppliers
$17.00/1g
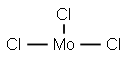
13478-18-7
62 suppliers
$25.00/1G
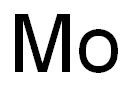
7439-98-7
301 suppliers
$17.00/1g