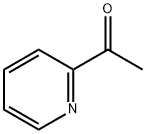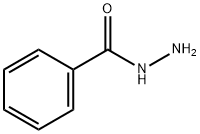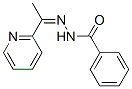
N'-[1-(2-Pyridinyl)ethylidene]benzohydrazide synthesis
- Product Name:N'-[1-(2-Pyridinyl)ethylidene]benzohydrazide
- CAS Number:1219-42-7
- Molecular formula:C14H13N3O
- Molecular Weight:0
Yield:1219-42-7 95%
Reaction Conditions:
at 20; for 4 h;Milling;Green chemistry;
Steps:
2.2. Green synthesis of Schiffbase ligand (HL)
Mixing benzohydrazide (0.01 mol; 1.36 g) and 2-acetylpyridine (0.01 mol; 3.06 g) into a ball-mill vessel consisting of Retsch MM2000 stainless steel and containing steel balls was used to make the N-(1-(pyridine-2-yl)ethylidene)benzohydrazide (HL) (10.0 g) ligand. As a result, the device must be tightly sealed, and the grinding process should begin at room temperature. The quality of the reaction was ensured by analyzing the products using thin layer chromatography (TLC) every 5 min until it was completed. After 30 min, this accuracy was achieved, with no residues of reactants contaminating the products ( Scheme 1 ). HL (1); (white color): Mw; 239.27; Elm. Anal. for C14H13N3O (m.p. 125 °C, Yield: 95%); Calcd: C, 70.3; H, 5.5%. Found: C, 70.21; H, 5.52. IR (KBr, cm -1 )(Fig S1), (C = N) py 1631, (C = N) azo 1661, (C = O) 1685, (NH) 3233. 1 H NMR (500 MHz, DMSO-d6) (Fig. S2), (ppm): 2.60 (s, 3H, CH3), 7.47 (t, 1H, H5), 7.91 (t, 2H, H4), 8.22 (d, 1H, H3), 8.38 (t, 2H, H10, 14), 8.58 (d, 2H, H11, 13), 8.64 (d, 1H, H6), 10.81 (s,1H, NH). 13 C NMR (DMSO-d 6) (Fig. S3): (ppm) 149.14, 120.33, 136.61, 124.25, 134.57, 154.76, 162.68, 134.16, 139.61, 129.52 (2C), 123.30 (2C). It’s worth noting that the number for hydrogen atoms was revealed in Scheme 1 over the structure.
References:
Abumelha, Hana M.;Alatawi, Nada M.;Alaysuy, Omaymah;Alharbi, Arwa;Alsoliemy, Amerah;El-Metwaly, Nashwa M.;Osman, Hanan E. M.;Zaky, Rania [Journal of Molecular Structure,2022,vol. 1259,art. no. 132748]
93-58-3
465 suppliers
$14.00/25mL
![N'-[1-(2-Pyridinyl)ethylidene]benzohydrazide](/CAS/GIF/1219-42-7.gif)
1219-42-7
0 suppliers
inquiry
93-89-0
504 suppliers
$13.00/100g
![N'-[1-(2-Pyridinyl)ethylidene]benzohydrazide](/CAS/GIF/1219-42-7.gif)
1219-42-7
0 suppliers
inquiry