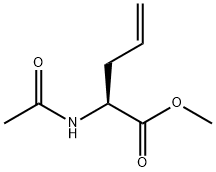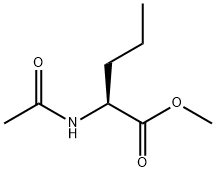
N-acetylnorvaline methyl ester synthesis
- Product Name:N-acetylnorvaline methyl ester
- CAS Number:1492-17-7
- Molecular formula:C8H15NO3
- Molecular Weight:173.21
Yield:95 % ee ,1492-17-7 3%
Reaction Conditions:
with hydrogen;(+)-1,2-bis[(2S,5S)-2,5-diethylphospholano]benzene(1,5-cyclooctadiene)rhodium(I) trifluoromethane-sulfonate in benzene at 22; under 1551.49 Torr; for 3 h;Product distribution / selectivity;
Steps:
7.7.2
The dienamide 57 was subjected to the general asymmetric hydrogenation procedure (Section 7.4.3) under the following conditions: (2Z)-Methyl 2-N-acetylaminopenta-2,4-dienoate 57 (108 mg, 0.64 mmol), benzene (7 mL), Rh(I)-(S,S)-Et-DuPHOS, 30 psi, 22° C., 3 h. At the end of the reaction period, the solvent was evaporated under reduced pressure and the residue was purified by flash chromatography (SiO2, EtOAc) to give a yellow oil (106 mg, 97%). 1H n.m.r. spectroscopy confirmed formation of the desired product 21a and the fully saturated compound, (2S)-methyl 2-N-acetylaminopentanoate 59 (δ 0.93 (t, J=7.3 Hz, 3H, H5), 1.25-1.44 (m, 4H, H3, 4)), in a 97:3 ratio respectively. GC: (2S)-21a tR=18.6 min (GC chiral column 50 CP2/XE60-SVALSAPEA, 100° C. for 1 min, 5° C. min-1 to 280° C. for 9 min), 95% e.e. [α]D22+45.0° (c=0.76, CHCl3) containing 3% of 59 (lit.208 for (S)-21a[α]D22+45.4° (c=3.57, CHCl3)). νmax (neat): 3278s, 3079w, 2955w, 1744s, 1657s, 1546m, 1438m, 1375m, 1275w, 1226w, 1151m, 997w, 924w cm-1. 1H n.m.r. (300 MHz, CDCl3): δ 2.00 (s, 3H, CH3CO), 2.43-2.62 (m, 2H, H3), 3.73 (s, 3H, OCH3), 4.67 (dt, J=11.6, 5.8 Hz, 1H, H2), 5.08 (m, 1H, H5-E), 5.14 (m, 1H, H5-Z), 5.67 (m, 1H, H4), 6.06 (bs, 1H, NH). 13C n.m.r. (100 MHz, CDCl3): δ 23.1 (CH3CO), 36.5 (C3), 51.8 (C2), 52.4 (OCH3), 119.2 (C5), 132.3 (C4), 169.9, 172.4 (C1, CONH). Mass Spectrum (ESI+, MeOH): m/z 194.1 (M+Na)+, C8H13NNaO3 requires 194.2. Spectroscopic data were in agreement with those reported in the literature.119
References:
US2007/197429,2007,A1 Location in patent:Page/Page column 57; 54; 11