
N-Boc-N'-Fmoc-L-Lysine synthesis
- Product Name:N-Boc-N'-Fmoc-L-Lysine
- CAS Number:84624-27-1
- Molecular formula:C26H32N2O6
- Molecular Weight:468.54
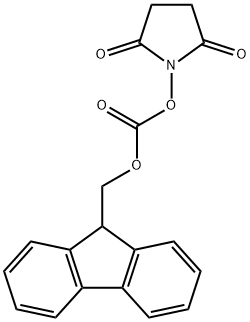
82911-69-1
599 suppliers
$7.00/25g
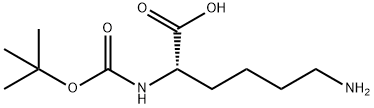
13734-28-6
345 suppliers
$8.00/1g

84624-27-1
259 suppliers
$6.00/1g
Yield:-
Reaction Conditions:
with sodium hydrogencarbonate in tetrahydrofuran;water at 20; for 3 h;
Steps:
53.1 Step 1 : Preparation of (S)-6-((((9H-fluoren-9-yl)methoxy)carbonyl)amino)-2-((tert- butoxycarbonyl)amino)hexanoic acid (intermediate i-53a)
To a solution of (2S)-6-amino-2-(tert-butoxycarbonylamino)hexanoic acid (1 g, 4.06 mmol) in THF (10.0 mL) was added a solution of NaHCC>3 (682.14 mg, 8.12 mmol) in water (10.0 ml_) and FmocOSu (1 .51 g, 4.47 mmol). The reaction mixture was stirred at 20 °C for 3 hrs. The reaction mixture was poured into water (10.0 mL). The solution was adjusted to pH4 with saturated citric acid solution. The mixture was extracted with EtOAc (15 mL*3). The combined organic extracts were washed with brine (20.0 mL), dried over Na2SC>4 and filtered. The filtrate was concentrated to give intermediate i-53a as a white solid. The compound was used directly in the next step. LCMS (ESI) m/z: [M+Na]+ = 491 .4
References:
FOGHORN THERAPEUTICS INC.;ANTHONY, Neville, John;MILLAN, David, Simon;VASWANI, Rishi, G.;SCHILLER, Shawn, E.R. WO2019/152437, 2019, A1 Location in patent:Page/Page column 133-134

133628-28-1
47 suppliers
$41.00/250mg

84624-27-1
259 suppliers
$6.00/1g
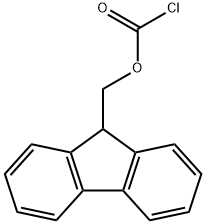
28920-43-6
523 suppliers
$5.00/5g

13734-28-6
345 suppliers
$8.00/1g

84624-27-1
259 suppliers
$6.00/1g

80994-44-1
0 suppliers
inquiry
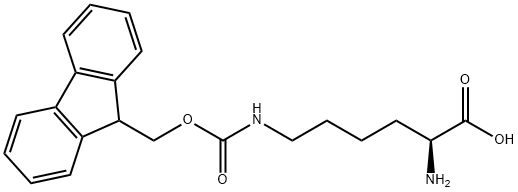
84624-28-2
138 suppliers
$17.30/250mg

84624-27-1
259 suppliers
$6.00/1g