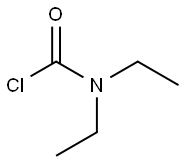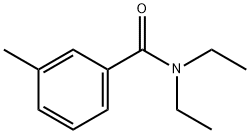
N,N-Diethyl-m-toluamide synthesis
- Product Name:N,N-Diethyl-m-toluamide
- CAS Number:134-62-3
- Molecular formula:C12H17NO
- Molecular Weight:191.27
Yield:134-62-3 97.5%
Reaction Conditions:
with triethylamine at 20; for 0.333333 h;
Steps:
2 Example 2. Preparation of Ν,Ν-Diethyl m-toluamide (DEET)
136 g ( 1 Mole) of m-toluic acid (3-methyl benzoic acid) and 136 g (= 1 27 ml. 1 Mole) N,N-diethylcarbamoyl chloride are taken in a 1 liter two-necked round-bottom flask lltted with air condenser which is placed over a magnetic stirrer. To this, 121 g ( 167 ml. 1.2 Mole) of triethylamine, which is a organic base is added using a pressure-equalizing funnel fitted in the side neck of the round bottom flask at room temperature. After complete addition, the reaction m ixture is stirred constantly for 20 minutes at room temperature. The reaction mixture is then treated with 250 ml of water and the two layers are separated. Pure and colourless N,N-diethyl m-toluamide (DEET) is obtained by vacuum disti l lation of organic layer wh ich is the product. Purity of the compound is analyzed using GC-MS which is more than 99.5%. The yield of the product is 186 g (97.5%).
References:
WO2013/65059,2013,A1 Location in patent:Page/Page column 13
201230-82-2
1 suppliers
inquiry
625-95-6
251 suppliers
$14.00/25g

109-89-7
449 suppliers
$10.00/5g

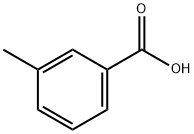
134-62-3
480 suppliers
$14.00/25g

109-89-7
449 suppliers
$10.00/5g
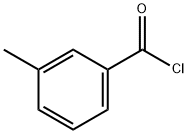
1711-06-4
304 suppliers
$6.00/25g

134-62-3
480 suppliers
$14.00/25g

617-84-5
240 suppliers
$25.00/25mL
99-04-7
438 suppliers
$8.00/100g

134-62-3
480 suppliers
$14.00/25g