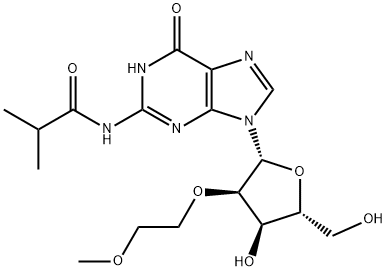
N2-iso-Butyroyl-2'-O-(2-methoxyethyl)guanosine synthesis
- Product Name:N2-iso-Butyroyl-2'-O-(2-methoxyethyl)guanosine
- CAS Number:440327-50-4
- Molecular formula:C17H25N5O7
- Molecular Weight:411.41
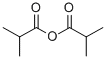
97-72-3
215 suppliers
$10.00/10g
473278-54-5
114 suppliers
$33.00/100mg

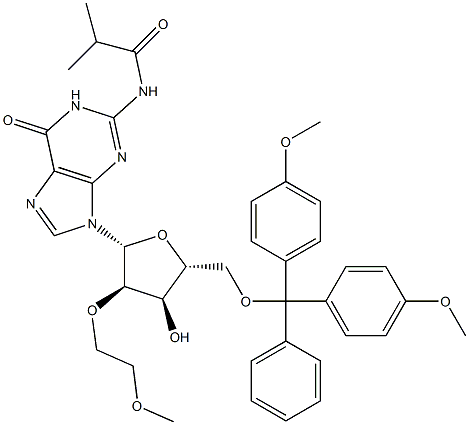
440327-50-4
37 suppliers
inquiry
Yield:440327-50-4 106.6 g
Reaction Conditions:
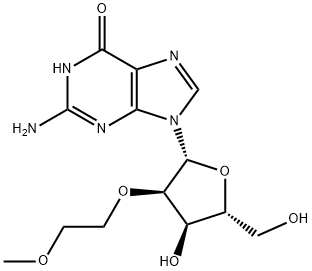
Stage #1: 2'-O-(2-methoxyethyl)-guanosinewith pyridine;chloro-trimethyl-silane in dichloromethane at 0 - 25; for 2 h;
Stage #2: 2-Methylpropionic anhydride in dichloromethane at 0 - 25; for 0.5 h;
Stage #3: with ammonium hydroxide in dichloromethane;water at 20 - 25; for 12.5 h;
Steps:
2 Synthesis of 2-2
To the 2’-O-Methoxyethyl-guanosine (2-1, 100g) in flask was added pyridine (400mL) and CH2Cl2(600mL). After cooling flask to 0-5 °C, TMSCl (3.5 eq.) was added dropwise into reaction solution. Reaction solution was stirred at 25 °C for 2h. The reaction mixture was cooled to 0-5°C. Isobutyric anhydride (2 eq.) was added into reaction. The mixture was stirred at 25 °C for 0.5h. The mixture was processed: water was added into flask below 20 °C. aq. NH4OH was added into flask below 20 °C. The mixture was stirred at 20-25 °C for 30min. The mixture was stirred at 25°C for another 12h. The aqueous layer was separated and extracted with DCM:MeOH=10:1. The organic layers were separated and combined. The combined organic layers were concentrated under reduced pressure. After addition of toluene, the mixture was concentrated under vacuum. After addition of 1,1,1,3,3,3-Hexafluoro-2-Propanol (HFIP) into flask dropwise at 0°C, MTBE was added into the solution dropwise at 25°C. Crystallization was observed immediately. The mixture was stirred at 25°C for 1h. The mixture was filtered and washed filter cake with 300mL MTBE. The filter cake was collected and dried at 40°C under vacuum to afford 2-2 as a white solid 106.6g, 58.5% (yield corrected by assay).1H-NMR (400MHz, d6-DMSO) δ12.10 (bs, 1H), 11.68 (bs, 1H), 8.29 (s, 1H), 5.90 (d, J=6.4Hz, 1H), 5.14 (d, J=4.4Hz, 1H), 5.08 (t, J=5.6Hz, 1H), 4.42-4.41 (m, 1H), 4.30-4.29 (m, 1H), 4.19- 4.11 (m, 1H), 3.94-3.93 (m, 1H), 3.68-3.53 (m, 4H), 3.16 (s, 3H), 2.79-2.75 (m,1H), 1.12 (d, J=6.8Hz, 6H).13C-NMR (100MHz, d6-DMSO): 180.6, 155.3, 149.3, 148.7, 138.0, 120.4, 86.5, 84.9, 82.0, 71.5, 69.3, 61.6, 58.4, 35.2, 19.2. LC-MSESIm/z: found 412 [M+H]+, 410 [M-H]-
References:
WO2021/186328,2021,A1 Location in patent:Paragraph 0088
251647-50-4
64 suppliers
$60.00/50mg

440327-50-4
37 suppliers
inquiry
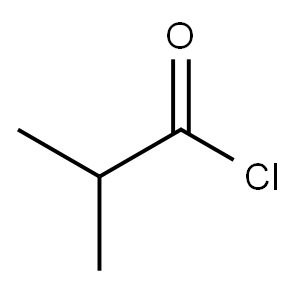
79-30-1
359 suppliers
$10.00/10g

473278-54-5
114 suppliers
$33.00/100mg

440327-50-4
37 suppliers
inquiry
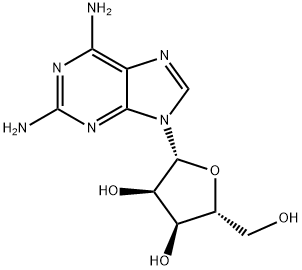
2096-10-8
279 suppliers
$5.00/1g

440327-50-4
37 suppliers
inquiry
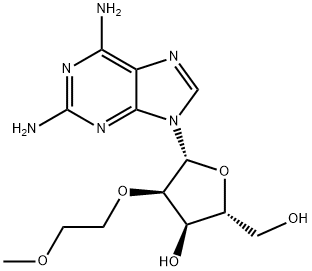
256224-13-2
19 suppliers
inquiry

440327-50-4
37 suppliers
inquiry