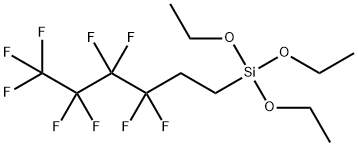
Nonafluorohexyltriethoxysilane synthesis
- Product Name:Nonafluorohexyltriethoxysilane
- CAS Number:102390-98-7
- Molecular formula:C12H19F9O3Si
- Molecular Weight:410.35
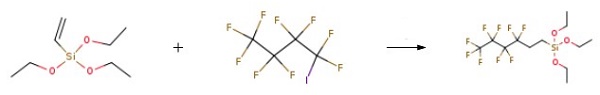
78-08-0
272 suppliers
$11.19/100G
423-39-2
231 suppliers
$6.00/5g

102390-98-7
81 suppliers
$17.00/5g
Yield:102390-98-7 75%
Reaction Conditions:
Stage #1: Triethoxyvinylsilane;1-iodo-2,2,3,3,4,4,5,5,5-nonafluorobutanewith 2,2'-azobis(isobutyronitrile) at 60; for 6 h;
Stage #2: with tri-n-butyl-tin hydride at 25; for 4 h;
Steps:
4
Example-4: Nonafluorobutyl iodide (NFBI) and triethoxyvinylsilane (TEOVS) were fed into a three-necked flask equipped with a reflux condenser at a molar ratio of 1: 1.1, and then heated to 60 DEG C with stirring. When the internal temperature of the flask was sufficiently heated, azobisbutylnitrile (AIBN), which is a thermal decomposition radical initiator, was dissolved in an ether-based solvent, and then 0.5 ml / sec was dropped slowly to the stirred flask. Thereafter, when the dropwise addition was completed, the external heating temperature was again set to 60 DEG C and stirred for 6 hours. Thereafter, the internal temperature of the flask was lowered to room temperature (25 DEG C), and tributyltin hydride (TBTH) was added to the flask at a rate of 1 ml / sec. After further stirring for an additional 4 hours, distillation under reduced pressure at 1 Torr and 40 ° C yielded 1H, 2H, 2H-Perfluorohexyltriethoxysilane in 75% yield.
References:
KR2016/77963,2016,A Location in patent:Paragraph 0107; 0108