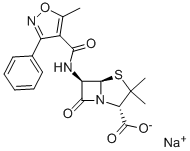
OXACILLIN SODIUM synthesis
- Product Name:OXACILLIN SODIUM
- CAS Number:1173-88-2
- Molecular formula:C19H18N3NaO5S
- Molecular Weight:423.42
551-16-6
488 suppliers
$7.00/10g
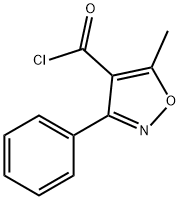
16883-16-2
159 suppliers
$20.00/5g

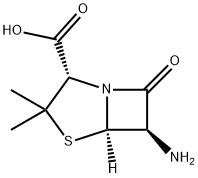
1173-88-2
258 suppliers
$11.00/1g
Yield:-
Reaction Conditions:
with ammonium hydroxide in ethyl acetate; pH=2 - 9
Steps:
4
Benzaldehyde (850g) is dissolved in methanol (4-5 L) at room temperature. Hydroxylamine salt (516-550 g) was added and the reaction mixture was heated to 38-40aC for 1 hour followed by addition of sodium carbonate (approx450-500g). The pH is maintained at around 9.5 and the reaction mixture is cooled to room temperature. Triethylamine (4g) is added and chlorine gas (510-520g) is passed for 24hrs by maintaining a temperature of 0-5aC. Nitrogen gas is purged for 4-5 hrs. After the completion of the chlorination reaction, methanol (approx. l OOOmL) is added followed by addition of sodium carbonate (425-450g) to reach a pH of around 7-7.5. Methylacetoacetate (686gapp) and soda ash (25-50g) is added to the reaction mixture till an exothermic reaction is achieved. The solution thus obtained was saponified by the addition of caustic flakes (approx. 460g). Sulphuric acid was added and the pH was adjusted to around 8.6-8.8. Organic impurities are extracted in solvent , acid is isolated dried , added in Toluene(about 3.5 Its) Phosphorous pentachloride (approx540g) was added with constant stirring. After reaction is over, organic layer is washed with water , Toluene recovered under reduced pressure. To this resulting solution of 3-phenyl-5-methyl-4- isoxazole-carbonyl chloride, ethyl acetate was added. 6-aminopenicillanic acid (300g) was dissolved in ammonium hydroxide (260-270ml_) and pH was adjusted to 7.0-9.0. This solution was then added to the reaction solution containing 3-(2-chlorophenyl)-5-methyl-4- isoxazole-carbonyl chloride (336g) and the pH was maintained to be around 2.0-2.3. Common salt (100g) was added with constant stirring and the layers were allowed to settle. Organic layers were separated and the combined extracts were then washed with brine followed by addition of sodium-2-ethyl hexanoate (590-630g) while maintaining the pH at 7.8- 8.1 . The solution was then agitated and cooled to 0 to 5aC. The resulting precipitate was filtered under reduced pressure , washed with organic solvent and nitrogen gas atmosphere. The wet precipitate was then subject to vacuum drying to yield sodium salt of 6-[[3-phenyl-5- methyl-1 ,2-oxazole-4-carbonyl]amino]-3,3-dimethyl-7-oxo-4-thia-1 -azabicyclo[3.2.0]heptane- 2-carboxylic acid (oxacillin sodium).
References:
VARDHMAN CHEMTECH LIMITED;GUJRAL, Rajinder Singh;JAIN, Suyog WO2012/164355, 2012, A1 Location in patent:Page/Page column 12