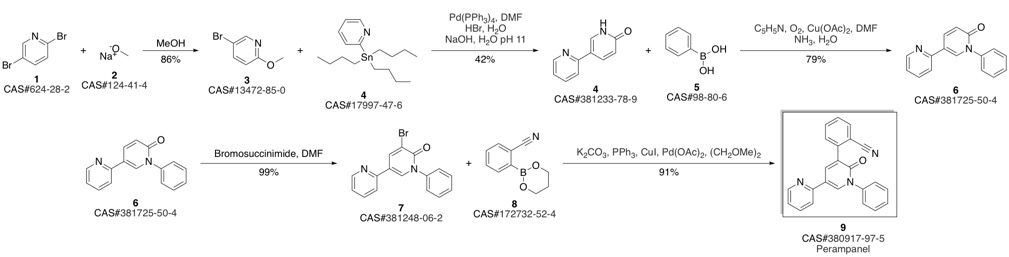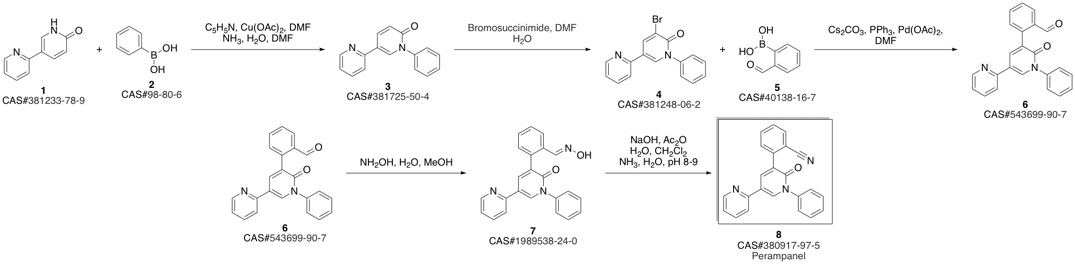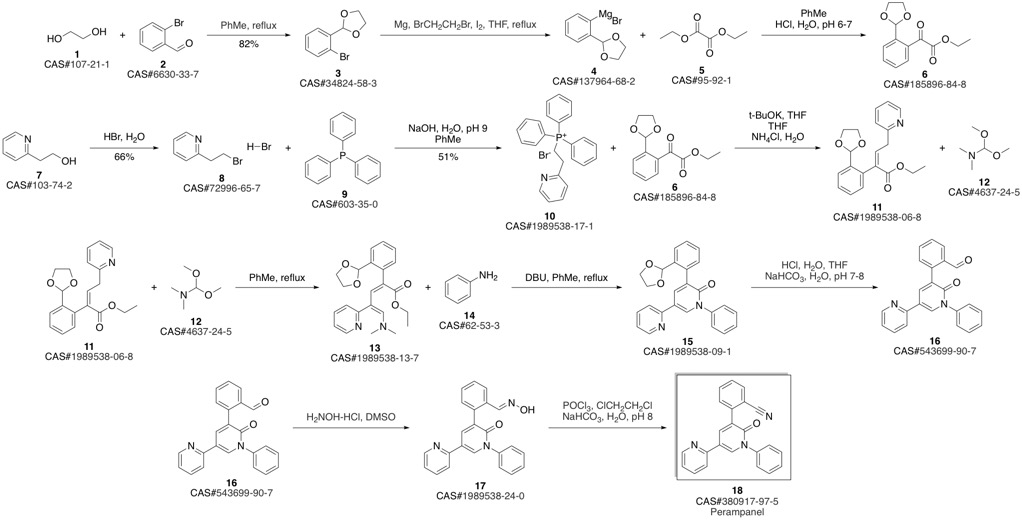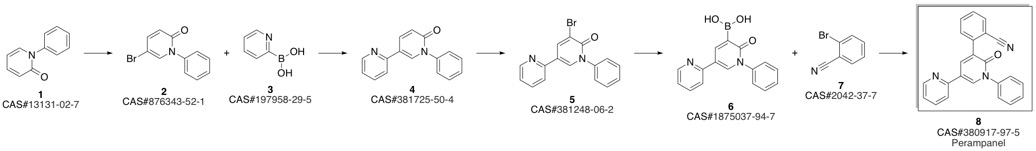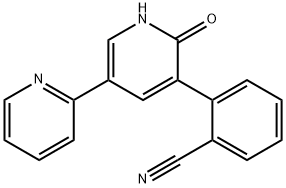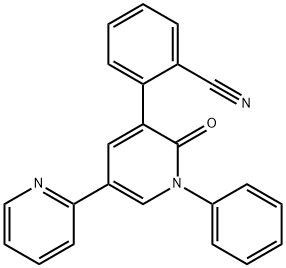
PeraMpanel synthesis
- Product Name:PeraMpanel
- CAS Number:380917-97-5
- Molecular formula:C23H15N3O
- Molecular Weight:349.38
: Hibi, Shigeki; Ueno, Koshi; Nagato, Satoshi; Kawano, Koki; Ito, Koichi; Norimine, Yoshihiko; Takenaka, Osamu; Hanada, Takahisa; Yonaga, Masahiro. Discovery of 2-(2-Oxo-1-phenyl-5-pyridin-2-yl-1,2-dihydropyridin-3-yl)benzonitrile (Perampanel): A Novel, Noncompetitive α-Amino-3-hydroxy-5-methyl-4-isoxazolepropanoic Acid (AMPA) Receptor Antagonist. Journal of Medicinal Chemistry. Volume 55. Issue 23. Pages 10584-10600. Journal; Online Computer File. (2012).
![5'-broMo-1'-phenyl-[2,3'-bipyridin]-6'(1'H)-one](/CAS/GIF/381248-06-2.gif)
381248-06-2
86 suppliers
$34.00/100mg
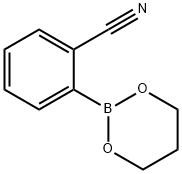
172732-52-4
242 suppliers
$19.00/1g

380917-97-5
142 suppliers
$79.00/5mg
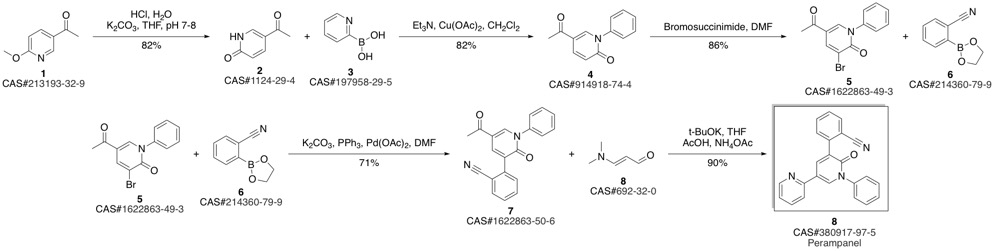
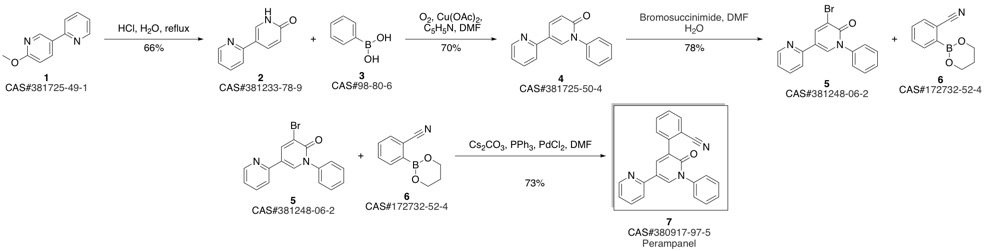
Yield:380917-97-5 73%
Reaction Conditions:
with caesium carbonate;palladium dichloride in N,N-dimethyl-formamide at 80; for 6 h;Inert atmosphere;Suzuki-Miyaura Coupling;
References:
Hibi, Shigeki;Ueno, Koshi;Nagato, Satoshi;Kawano, Koki;Ito, Koichi;Norimine, Yoshihiko;Takenaka, Osamu;Hanada, Takahisa;Yonaga, Masahiro [Journal of Medicinal Chemistry,2012,vol. 55,# 23,p. 10584 - 10600]
![Benzonitrile, 2-(1',6'-dihydro-6'-oxo-1'-phenyl[2,3'-bipyridin]-5'-yl)-, hydrate (1:1)](/CAS/20211123/GIF/942063-28-7.gif)
942063-28-7
0 suppliers
inquiry

380917-97-5
142 suppliers
$79.00/5mg
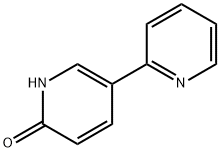
381233-78-9
165 suppliers
$9.00/100mg

380917-97-5
142 suppliers
$79.00/5mg
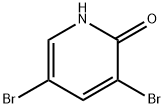
13472-81-6
199 suppliers
$9.00/1g

380917-97-5
142 suppliers
$79.00/5mg