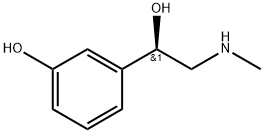
Phenylephrine synthesis
- Product Name:Phenylephrine
- CAS Number:59-42-7
- Molecular formula:C9H13NO2
- Molecular Weight:167.21
![2-Naphthaleneacetic acid, 6-methoxy-α-methyl-, (αR)-, compd. with (αR)-3-hydroxy-α-[(methylamino)methyl]benzenemethanol (1:1)](/CAS/20211123/GIF/1280541-42-5.gif)
1280541-42-5
0 suppliers
inquiry

59-42-7
192 suppliers
$23.30/1G
Yield:59-42-7 90%
Reaction Conditions:
Stage #1: (R)-phenylephrine-(R)-naproxenwith hydrogenchloride in water;toluene at 75 - 80; pH=< 2; for 0.5 h;
Stage #2: with ammonium hydroxide in water at 1 - 15; pH=~ 9;Product distribution / selectivity;
Steps:
2.b
(b) The phenylephrine-R-naproxen salt (62.5 g) was suspended in 75 mL of purified water, pH adjusted to <2 with HCl (12 mL) at 25-35° C. and charged with 208 mL of toluene. The suspension was warmed to 75-80° C. and maintained for 30 minutes. The reaction mass separated into two layers. The upper toluene layer separated and the bottom aqueous layer was extracted with 55 mL of toluene at 75-85° C. The toluene extracts were combined and reserved for recovery of R-naproxen. The aqueous layer was cooled to about 15° C. and the pH adjusted to about 9 with ammonium hydroxide solution (20 mL). The reaction mixture was further cooled to 1-5° C. and stirred for about 1 hr at 1 to 5° C. The crystalline R-phenylephrine base obtained was filtered, washed with 25 mL of purified water and dried under vacuum at 75° C. until moisture content was <0.2%. Yield 18.4 g (90%), m.r.: 170-180° C., OR: -30 to -35° (c=2% in MeOH).
References:
US2012/108848,2012,A1 Location in patent:Page/Page column 4

614-03-9
8 suppliers
inquiry

59-42-7
192 suppliers
$23.30/1G
94240-17-2
92 suppliers
$110.00/10mg

59-42-7
192 suppliers
$23.30/1G
![Benzenemethanol, 3-methoxy-α-[(methylamino)methyl]-, (αR)-](/CAS/20210305/GIF/747358-03-8.gif)
747358-03-8
0 suppliers
inquiry

59-42-7
192 suppliers
$23.30/1G