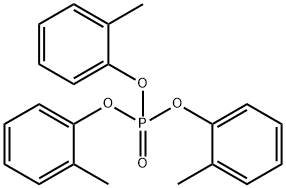
PHOSPHOROUS ACID TRI-O-CRESYL ESTER synthesis
- Product Name:PHOSPHOROUS ACID TRI-O-CRESYL ESTER
- CAS Number:2622-08-4
- Molecular formula:C21H21O3P
- Molecular Weight:352.36
95-48-7
434 suppliers
$17.00/25g

2622-08-4
61 suppliers
$64.06/1G

22277-50-5
0 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
Yield: 7 - 14 %Spectr. , 14 - 68 %Spectr. , 18 - 58 %Spectr.
Reaction Conditions:
with triethylamine;phosphorus trichloride in toluene at -5; for 3 - 6.66667 h;
Steps:
A; B; C COMPARATIVE EXAMPLES Comparative Example A
Synthesis of phosphorochloridite 1a and phosphorodichloridite 2a under the conditions described in where a mixture of amine and alcohol was added to the PCl3 at 0.67 equiv/h [0050] A temperature controlled 250 ml baffled flask was charged with 50 ml of 1.0 molar PCl3 in toluene. Under vigorous stirring a mixture of 25 ml 2.0 molar triethylamine in toluene and 25 ml 2.0 molar o-cresol in toluene was added via a single syringe pump over a period of 90 min. During the addition period the reaction temperature was maintained at -5° C. The 31P NMR analysis showed transformation to the corresponding dichloridite 2a in only 16% selectivity. The distribution was PCl3 56%; dichloridite 7%; chloridite 9%; triarylphosphite 28%. Another mixture of 25 ml 2.0 molar triethylamine in toluene and 25 ml 2.0 molar o-cresol in toluene was added under vigorous stirring via a single syringe pump over a period of 90 min. During the addition period the reaction temperature was maintained at -5° C. The 31P NMR analysis showed transformation to the corresponding chloridite 1a in only 14% overall selectivity. The distribution was PCl3 21%; dichloridite 7%; chloridite 14%; triarylphosphite 58%.Comparative Example B [0051] Synthesis of a phosphorochloridite 1a and phosphorodichloridite 2a under the conditions described in where a mixture of amine and alcohol are added to the PCl3 at 0.3 equiv/h. [0052] A temperature controlled 300 ml baffled round bottom flask was charged with 25 ml of 2.0 molar PCl3 in toluene. Under vigorous stirring a mixture of 25 ml 2.0 molar triethylamine in toluene and 25 ml 2.0 molar o-cresol in toluene was added via a single syringe pump over a period of 200 min. During the addition period the reaction temperature was maintained at -5° C. The 31P NMR analysis showed transformation to the corresponding dichloridite 2a in only 14% selectivity. The distribution was PCl3 57%; dichloridite 6%; chloridite 10%; and triarylphosphite 28%. Another mixture of 25 ml 2.0 molar triethylamine in toluene and 25 ml 2.0 molar o-cresol in toluene was added under vigorous stirring via a single syringe pump over a period of 200 min. During the addition the reaction temperature was maintained at -5° C. The 31P NMR analysis showed transformation to the corresponding chloridite 1a in only 17% overall selectivity. The distribution was PCl3 18%; dichloridite 9%; chloridite 17%; and triarylphosphite 56%.Comparative Example C [0053] Synthesis of phosphorochloridite 1a and phosphorodichloridite 2a where two equivalents of the amine were added to a mixture of one equivalent of PCl3 and two equivalents of alcohol. [0054] A temperature controlled 300 ml baffled round bottom flask was charged with a mixture of 50 ml of 1.0 molar PCl3 and 50 ml of 2.0 molar o-cresol both in toluene. A solution of 25 ml 2.0 molar triethylamine in toluene was added under vigorous stirring via a single syringe pump over a period of 100 min. During the addition period the reaction temperature was maintained at -5° C. The 31P NMR analysis showed transformation to the corresponding dichloridite 2a in only 51% selectivity. The distribution was PCl3 10%; dichloridite 46%; chloridite 29%; triarylphosphite 15%. Another 25 ml 2.0 molar triethylamine in toluene were added under vigorous stirring via a single syringe pump over a period of 100 min. During the addition period the reaction temperature was maintained -5° C. The 31P NMR analysis showed transformation to the corresponding chloridite 1a in only 68% overall selectivity. The distribution was dichloridite 14%, chloridite 68%, and triarylphosphite 18%.
References:
Ritter, Joachim C. US2004/106815, 2004, A1 Location in patent:Page 12-13

95-48-7
434 suppliers
$17.00/25g

2622-08-4
61 suppliers
$64.06/1G