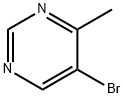
Pyrimidine, 5-bromo-4-methyl- (7CI,8CI,9CI) synthesis
- Product Name:Pyrimidine, 5-bromo-4-methyl- (7CI,8CI,9CI)
- CAS Number:1439-09-4
- Molecular formula:C5H5BrN2
- Molecular Weight:173.01
4595-59-9
457 suppliers
$6.00/1g
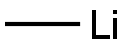
917-54-4
174 suppliers
$44.39/50ml

1439-09-4
126 suppliers
$39.00/250mg
Yield: 15%
Reaction Conditions:
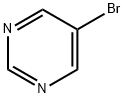
Stage #1:5-bromopyrimidine;methyllithium in diethyl ether at 20; for 1 h;
Stage #2: with water;2,3-dicyano-5,6-dichloro-p-benzoquinone in tetrahydrofuran;diethyl ether at 20; for 16 h;
Steps:
48
To 5-bromopyrimidine (17.3 g, 109 mmol) in diethyl ether (100 mL) , methyllithium in diethyl ether (109 mmol, 1.09 M, 100 mL) was gradually added dropwise at room temperature, and the resulting reaction mixture was stirred at room temperature for 1 hour. After completion of the reaction, the reaction mixture was stirred with water (1.96 mL, 109 mmol) and 2 , 3-dichloro-5 , 6-dicyano-p- benzoquinone (24.7 g, 109 mmol) in tetrahydrofuran (150 mL) at room temperature for 16 hours. After completion of the reaction, water and ethyl acetate were added, and the organic layer was separated. The organic layer was washed with IM aqueous sodium hydroxide, dried over anhydrous magnesium sulfate and evaporated under reduced pressure. The resulting residue was purified by silica gel column chromatography (hexane/ethyl acetate = 1/1) to give the desired product (2.9 g, 15% yield) . Morphology: yellow oil 1H-NMR(CDCl3) δ :2.65 (s, 3H), 8.72 (s, 1H), 8.98 (s, 1H)
References:
NISSAN CHEMICAL INDUSTRIES, LTD. WO2009/57827, 2009, A1 Location in patent:Page/Page column 167

3438-55-9
97 suppliers
$36.00/100mg

1439-09-4
126 suppliers
$39.00/250mg