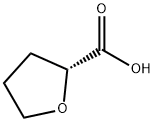
(R)-(+)-2-Tetrahydrofuroic acid synthesis
- Product Name:(R)-(+)-2-Tetrahydrofuroic acid
- CAS Number:87392-05-0
- Molecular formula:C5H8O3
- Molecular Weight:116.12
16874-33-2
282 suppliers
$6.00/5g
87392-07-2
264 suppliers
$10.00/1g

87392-05-0
347 suppliers
$14.00/1G
Yield:87392-05-0 9.339 g
Reaction Conditions:
with L-Phenylalaninol in ethyl acetate;acetone at 20; for 1 h;
Steps:
7; 9 Example 7:
Add L-phenylalaninol resolving agent (10g, 66mmol, 99% ee) to a 1000mL three-neck reaction flask and330 mL of acetone,After stirring and dissolving the dissolving agent,A mixture of 110 mL of ethyl acetate and (RS)-THFA (11.6 g, 100 mmol) was added.The reaction was mechanically stirred at 20 ° C for 1 h.A white solid precipitated, which was quickly filtered and weighed 7.536 g of diastereomer salt.The obtained diastereomer salt is recrystallized from acetone, first heated to about 56 ° C, to obtain a clear transparent liquid, stop heating, slowly cool naturally and stir at low speed until the temperature drops to 20 ° C, constant temperature and low speed stirring and crystallization for 1 h. Filtration, filter cake dissolved in 5mol / L sulfuric acid aqueous solution and adjusted to pH <2, adding DCM (3 × 100mL) extraction layering, liquid separation to obtain the first aqueous phase a1 and the first organic phase, combined with the first organic extract phase,Then dried over anhydrous Na2SO4, and concentrated to recover the solvent (S) -2-THFA (1.853g),99.71% ee, yield was 32%.The primary mother liquor and the recrystallization mother liquor obtained by the above filtration are combined, the solvent is distilled off, the distillation residue is fully dissolved with 5 mol/L sulfuric acid aqueous solution and adjusted to pH<2, and DCM (4×100 mL) is added for extraction and layering, and liquid separation is obtained. secondThe aqueous phase a2 and the second organic phase are combined with the extracted second organic phase,Then dried with anhydrous Na2SO4,The solvent (R)-2-THFA (9.339 g) was recovered by concentration.21.3% ee.
References:
CN109705064,2019,A Location in patent:Paragraph 0026; 0030; 0031
![Methanone, [(2S)-2-(hydroxydiphenylmethyl)-1-pyrrolidinyl][(2R)-tetrahydro-2-furanyl]-](/CAS/20210305/GIF/1152061-22-7.gif)
1152061-22-7
0 suppliers
inquiry

87392-05-0
347 suppliers
$14.00/1G
97-99-4
382 suppliers
$14.00/5g

87392-05-0
347 suppliers
$14.00/1G

97-99-4
382 suppliers
$14.00/5g

87392-07-2
264 suppliers
$10.00/1g

87392-05-0
347 suppliers
$14.00/1G
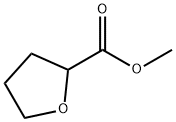
37443-42-8
140 suppliers
$18.00/5g

87324-01-4
9 suppliers
inquiry

87392-07-2
264 suppliers
$10.00/1g

87392-05-0
347 suppliers
$14.00/1G

87324-00-3
5 suppliers
inquiry