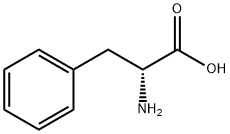
(R)-4-BENZYL-OXAZOLIDINE-2,5-DIONE synthesis
- Product Name:(R)-4-BENZYL-OXAZOLIDINE-2,5-DIONE
- CAS Number:37498-95-6
- Molecular formula:C10H9NO3
- Molecular Weight:191.18
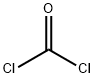
673-06-3
627 suppliers
$14.00/25G
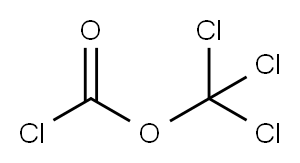
503-38-8
125 suppliers
$15.00/5g

37498-95-6
29 suppliers
$100.00/250mg
Yield:37498-95-6 89.7%
Reaction Conditions:
in tetrahydrofuran at 55; for 1.5 h;Temperature;
Steps:
General procedure for synthesis of NCAs
General procedure: A two-necked, round-bottomed flask was charged with the requisite amino acid (1 eq.) and dried under high vacuum at ~30-40 mTorr for >36 h to remove as much residual moisture as possible. The reaction flask was equipped with a reflux condenser topped with a dual-connection glass adapter, with one end connected to a nitrogen source and the other vented to the top of the fume hood (to properly exhaust the HCl vapors generated). Depending on the reaction scale, either a magnetic stir bar or mechanical paddle stirrer was inserted. Anhydrous THF was added to give an amino acid concentration of 0.4-0.5 M. To the resulting heavy suspension was added neat diphosgene (0.6-1.0 eq.; see Tables 2 and 4) in one portion. The reaction was carefully warmed to 55 °C with an oil bath or left to stir at ambient temperature. The reaction was considered complete when all the solids in the reaction dissolved and 1H NMR analysis of a vacuum-dried 0.3-mL aliquot indicated complete conversion to the product. Once at ambient temperature, the reaction mixture was transferred to a clean and dry round-bottomed flask, and concentrated on a rotary evaporator with the water bath maintained between 25 and 30 °C. Fresh anhydrous THF (~6-8 mL/g of amino acid) was added to dissolve the material before reconcentrating. The crude product was dissolved in a minimal amount of THF (~4-6 mL/g of amino acid) and transferred to a large precipitation container. Under a blanket of nitrogen, with vigorous mechanical stirring, heptane (6-8 crude volume) was added over 10-30 min to precipitate. The resulting solid was collected by vacuum filtration, washed with additional heptane (1-2 crude volume), and dried in a vacuum oven at room temperature overnight. Anhydrous DCM (~8-10 mL/g NCA) was added to the material and stirred for 15-30 min under nitrogen in order to dissolve as much of the crude material as possible. Oven-dried celite with a bed height of 2-4 cm was prepared in a sintered glass Buchner funnel, topped with a Whatman glass microfiber filter, and rinsed with anhydrous DCM before use (taking care to avoid cracks in the bed). The DCM-NCA suspension was filtered through the celite bed and then rinsed through with additional anhydrous DCM (3-5 mL/g of NCA). The clear filtrate was concentrated on a rotary evaporator with the water bath maintained at 25-30 °C. Anhydrous THF (~6-8 mL/g of NCA) was added to dissolve the product before reconcentrating. The material was dissolved in a minimal amount of THF (~4-6 mL/g of NCA), precipitated with heptane (6-8 crude volume), and collected/dried using the same method as described for the first precipitation. The final product was packaged under either nitrogen or argon in a container that was then sealed in a FoodSaver heat-seal vacuum bag and stored at 78 °C until use.
References:
Semple, J. Edward;Sullivan, Bradford;Sill, Kevin N. [Synthetic Communications,2017,vol. 47,# 1,p. 53 - 61]
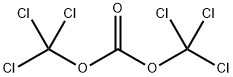
32315-10-9
441 suppliers
$10.00/1g

28069-46-7
3 suppliers
inquiry

37498-95-6
29 suppliers
$100.00/250mg