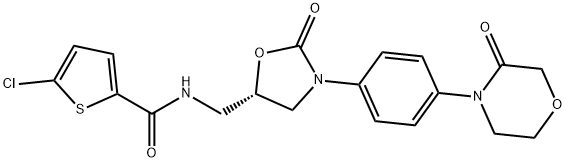
Rivaroxaban synthesis
- Product Name:Rivaroxaban
- CAS Number:366789-02-8
- Molecular formula:C19H18ClN3O5S
- Molecular Weight:435.88

Xu, Xin-liang; Li, Chao; Zhang, Xing-xian. Novel method for synthesis of anticoagulation drug rivaroxaban. Zhongguo Yaowu Huaxue Zazhi. Volume 24. Issue 2. Pages 90-93. Journal. (2014).
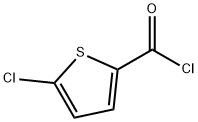
42518-98-9
271 suppliers
$8.00/1g
![3-Morpholinone, 4-[4-[(5S)-5-(aminomethyl)-2-oxo-3-oxazolidinyl]phenyl]-, methanesulfonate (1:1)](/CAS/20211123/GIF/1251952-62-1.gif)
1251952-62-1
0 suppliers
inquiry

366789-02-8
841 suppliers
$5.00/1mg
Yield:366789-02-8 96%
Reaction Conditions:
with potassium hydrogencarbonate in ethanol;water;butanone at 20 - 35; for 0.75 h;Large scale;
Steps:
Preparation of Rivaroxaban 1
The methanesulfonate salt (S)-12 (2.0 kg) was dissolved in a mixture of 5.0 L methylethyl ketone (MEK) and 10.0 L of water; we then added a solution of KHCO3 (1.55 kgin 5.0 L of water), and the mixture was stirred and cooled to 15 C. This was followedby the addition of a solution of the acid chloride of 5-chlorothiophene-2-carboxylic acid(13.9 L, 2.14 M in toluene) in 6.0 MEK and the reaction mixture was stirred at 20 Cfor 15 minutes. Then ethanol (16.0 L) was added and stirred at 35 C for 30 min. Themixture was stirred, heated to 50 C for 30 min, filtered, the clear filtrate cooled to 5 Cand stirred for 2 hours. The separated product was collected by filtration, washed withhot water (2 x 2.5 L, 60 C), ethanol (2 x 3.0 L) and dried under vacuum. Rivaroxaban(2.16 kg, yield 96%) was obtained in the form of a nearly white powder with m.p. 229.5-231 C, HPLC 99.95%, the content of (R)- isomer was under 0.03%. 1H NMR (DMSOd6),d(ppm): 3.61 (t, 2H, CH2); 3.71 (m, 2H, CH2); 3.85 a 4.19 (m, 2x1H, CH2); 3.97(m, 2H, CH2); 4.19 (s, 2H, CH2); 4.84 (pent, 1H, CH); 7.18 (d, 1H); 7.40 (m, 2H); 7.56(m, 2H); 7.68 (d, 1H); 8.95 (bt, 1H, NH).13C NMR (DMSO-d6), d(ppm): 42.2; 47.4;49.0; 63.4; 67.7; 71.3; 118.3; 125.9; 128.1; 128.4; 133.2; 136.4; 137.0; 138.4; 154.0; 160.8;165.9.MS (m/z): 436.0729 (MH).
References:
Halama, Ale?;Kruli?, Radim;Ryme?, Jan [Organic Preparations and Procedures International,2020]

42518-98-9
271 suppliers
$8.00/1g
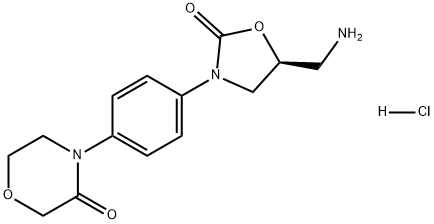
898543-06-1
316 suppliers
$8.00/250mg

366789-02-8
841 suppliers
$5.00/1mg
![3-MORPHOLINONE, 4-[4-[(5S)-5-(AMINOMETHYL)-2-OXO-3-OXAZOLIDINYL]PHENYL]-](/CAS/GIF/446292-10-0.gif)
446292-10-0
199 suppliers
$16.00/100mg

24065-33-6
730 suppliers
$6.00/5g

366789-02-8
841 suppliers
$5.00/1mg

1429333-90-3
1 suppliers
inquiry

366789-02-8
841 suppliers
$5.00/1mg

898543-06-1
316 suppliers
$8.00/250mg

1450877-56-1
49 suppliers
inquiry

366789-02-8
841 suppliers
$5.00/1mg








