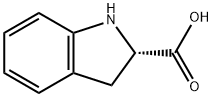
(S)-(-)-Indoline-2-carboxylic acid synthesis
- Product Name:(S)-(-)-Indoline-2-carboxylic acid
- CAS Number:79815-20-6
- Molecular formula:C9H9NO2
- Molecular Weight:163.17
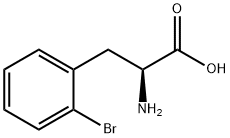
42538-40-9
165 suppliers
$13.00/250mg

79815-20-6
413 suppliers
$17.46/5G
Yield:79815-20-6 95.9%
Reaction Conditions:
with potassium carbonate;copper(l) chloride in 1-methyl-pyrrolidin-2-one at 80 - 100; for 3.5 - 4 h;Product distribution / selectivity;
Steps:
1; 2
Example 1 (S)-2,3-dihydro-1 H-indole-2-carboxylic acid: Conversion of (S)-2-bromophenylalanine with 2 mol% CuCl in NMP at 100°C A flask was charged successively with 366 mg (1.5 mmol) (S)-2-bromophenylalanine, 217 mg (1.6 mmol) K2CO3, 3.2 mg (0.03 mmol) CuCl and 3.2 g NMP. The reactor was flushed with argon and then kept under a slow stream of argon. The reaction mixture was stirred and heated until 100°C and kept at this temperature. Samples were taken regularly and analyzed by HPLC. After 4 hours, full conversion of (S)-2-bromophenylalanine was found. The yield (measured in solution) of (S)-2,3-dihydro-1 H-indole-2-carboxylic acid was 95.9 %, ee > 98.6%.; Example 2 (S)-2,3-dihydro-1H-indole-2-carboxylic acid: Conversion of (S)-2-bromophenylalanine with 1 mol% CuCl in NMP at 80°C A flask was charged successively with 9.76 g (40.0 mmol) (S)-2-bromophenylalanine, 5.80 g (42.0 mmol) K2CO3, 40 mg (0.4 mmol) CuCl and 40 g NMP. The reactor was flushed with argon and then kept under a slow stream of argon. The reaction mixture was stirred and heated until 80°C and kept at this temperature. Samples were taken regularly and analyzed by HPLC. After 3.5 h full conversion of (S)-2-bromophenylalanine was found. The reaction mixture was cooled to 25°C and then 40 mL H2O and 50 mL aqueous EtOAc were added. The pH of this mixture was adjusted to 3.3 with 3.5 g 37% aqueous HCl. The phases were separated. The H2O phase was extracted with 2 x 50 mL aqueous EtOAc. The combined organic layers were washed with 25 mL sat aqueous NaCl. Then the organic phase was concentrated. The residu was dissolved in 16 mL 5N aqueous HCl, followed by pH adjustment to 2.1 with 9.4 g 32% aqueous NaOH. The precipitated (S)-2,3-dihydro-1H-indole-2-carboxylic acid was isolated by filtration and washed with 2 x 10 mL H2O. 3.24 g (19.8 mmol) (S)-2,3-dihydro-1 H-indole-2-carboxylic acid was found after drying. Yield 49.5%, ee >99%.
References:
EP1676838,2006,A1 Location in patent:Page/Page column 16
1477-50-5
498 suppliers
$6.00/5g

79815-20-6
413 suppliers
$17.46/5G

103616-89-3
210 suppliers
$7.00/250mg

79815-20-6
413 suppliers
$17.46/5G

141410-06-2
41 suppliers
$45.00/100mg

79815-20-6
413 suppliers
$17.46/5G
![1H-Indole-2-carboxylic acid, 2,3-dihydro-, (2S)-, (1S,4R)-7,7-dimethyl-2-oxobicyclo[2.2.1]heptane-1-methanesulfonate (9CI)](/CAS/20211123/GIF/887769-80-4.gif)
887769-80-4
0 suppliers
inquiry

79815-20-6
413 suppliers
$17.46/5G