
SGI-1027 synthesis
- Product Name:SGI-1027
- CAS Number:1020149-73-8
- Molecular formula:C27H23N7O
- Molecular Weight:461.52
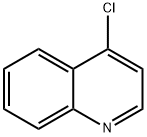
611-35-8
211 suppliers
$5.00/250mg

1020150-22-4
9 suppliers
inquiry

1020149-73-8
88 suppliers
$29.00/1mg
Yield: 0.045 g
Reaction Conditions:
with palladium diacetate;potassium carbonate;XPhos in water;N,N-dimethyl-formamide;tert-butyl alcohol at 110; for 24 h;Sealed tube;Inert atmosphere;Buchwald-Hartwig Coupling;
Steps:
N-[4-(2-Amino-6-methylpyrimidin-4-ylamino)phenyl]-4-(quinolin-4-ylamino)benzamide, SGI-1027 (4).
A sealed tube was charged with Pd(OAc)2 (1 mg, 1.32 mmol) and XPhos (2 mg, 3.97 mmol) and degassed t-BuOH/water (1 mL, 2:1 v/v) was added. The resulting solution was heated for 1 min at 80 ºC and then cannulated into another sealed tube charged with 4-amino-N-[4-(2-amino-6-methylpyrimidine-4-ylamino)phenyl]benzamide 12 (0.07g, 0.20 mmol), 4-chloroquinoline 13 (22.0 mg, 0.13 mmol) and potassium carbonate (0.03 g, 0.18 mmol) in DMF (1 mL). The resulting mixture was stirred for 24 h at 110 ºC. When the reaction was complete the solvent was concentrated in vacuo and the residue was purified by column chromatography (silica gel, 85:13:2 CH2Cl2/MeOH/Et3N) to afford 0.045 g (73% by 1H-NMR) of the titled compound as an amorphous yellow solid. 1H-NMR (400.13 MHz, DMSO-d6): d 10.22 (s, 1H, CONH), 9.98 (s, 1H, NH), 8.59 (d, J = 5.6 Hz, 1H), 8.48 (d, J = 8.4 Hz, 1H), 8.05 (d, J = 8.7 Hz, 2H), 8.00-7.94 (m, 2H), 7.86 - 7.75 (m, 3H), 7.74 - 7.61 (m, 4H), 7.57 - 7.50 (m, 3H), 7.44 - 7.37 (m, 1H), 7.15 (d, J = 5.6 Hz, 1H), 6.04 (s, 1H, H5’’), 2.22 (s, 3H, CH3) ppm. 13C-NMR (100.62 MHz, DMSO-d6): d 164.7 (s, CONH), 161.4 (s), 157.1 (s), 154.9 (s), 149.5 (s), 147.8 (d), 145.0 (s), 142.9 (s), 135.4 (s), 134.2 (s), 131.3 (d), 130.2 (s), 129.3 (d, 2x), 125.9 (d, 2x), 123.0 (d, 2x), 121.7 (d), 121.6 (d, 2x), 120.8 (d, 2x), 119.4 (s), 102.5 (d), 96.4 (d), 19.4 (q, CH3) ppm. HRMS (ESI+): Calcd for C27H24N7O ([M + H]+), 462.20368; found, 462.20330. IR (neat): n 3400-3000 (br, N-H), 2976 (w, C-H), 2891 (w, C-H), 1584 (m), 1510 (m), 1444 (m), 1380 (m), 1318 (m), 1228 (w), 1180 (w), 1095 (w) cm-1. UV (DMSO): lmax 258, 355 nm.
References:
García-Domínguez, Patricia;Dell'Aversana, Carmela;Alvarez, Rosana;Altucci, Lucia;De Lera, Ángel R. [Bioorganic and Medicinal Chemistry Letters,2013,vol. 23,# 6,p. 1631 - 1635] Location in patent:supporting information

5600-21-5
320 suppliers
$18.00/25g

1020149-73-8
88 suppliers
$29.00/1mg
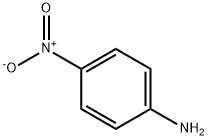
100-01-6
10 suppliers
$11.19/25G

1020149-73-8
88 suppliers
$29.00/1mg

20719-42-0
0 suppliers
inquiry

1020149-73-8
88 suppliers
$29.00/1mg

