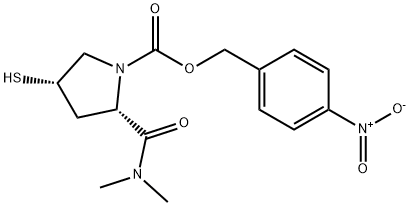
Side chain for meropenem synthesis
- Product Name:Side chain for meropenem
- CAS Number:96034-64-9
- Molecular formula:C15H19N3O5S
- Molecular Weight:353.39
![1,2-Pyrrolidinedicarboxylic acid, 4-mercapto-, 1-[(4-nitrophenyl)methyl] ester, (2S,4S)-](/CAS/20200611/GIF/127966-47-6.gif)
127966-47-6
0 suppliers
inquiry
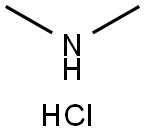
506-59-2
527 suppliers
$5.00/5G

96034-64-9
329 suppliers
$8.00/250mg
Yield:96034-64-9 92.3%
Reaction Conditions:
Stage #1: (2S,4S)-1-p-nitrobenzyloxycarbonyl-2-carboxy-4-mercaptopyrrolidinewith N-ethyl-N,N-diisopropylamine in N,N-dimethyl-formamide at 0 - 5; for 0.166667 h;
Stage #2: N,N-dimethylammonium chloride in N,N-dimethyl-formamide at 0 - 5; for 3 h;Concentration;
Steps:
10 Preparation of (2S, 4S) l-p-nitrobenzyloxycarbonyl-2- (N, N-dimethylcarbamoyl) -4-mercaptopyrrolidine (Formula VII)
The reaction flask was charged with 600 mL of DMF, and 179.5 g (0.55 mol) of (2S, 4S) -1- Carboxy-4-mercaptopyrrolidine (Formula VI) was prepared by reducing the temperature to 0 ° C to 5 ° C, adding 66.8 g (0.66 mol) Diisopropylethylamine, After stirring for 10 minutes, 89.7 g (1.10 mol) of dimethylamine hydrochloride was added and the mixture was stirred at 0 ° C to 5 ° C After 3 hours of reaction, 500 mL of purified ice water was quickly added, and a large amount of solid was precipitated under stirring. After filtration, the cake was washed with 200 mL of water , And dried under reduced pressure at 65 ° C for 15 hours to obtain 179.4 g of (2S, 4S) -1-p-nitrobenzyloxycarbonyl-2- (N, N-dimethylcarbamoyl Yl) -4-mercaptopyrrolidine (Formula VII), 92.3% molar yield, HPLC purity 99.3%. Compound VII mass spectrometry data are as follows: C15H19N3O5S, molecular weight: 353.4, [M + H] & lt; + & gt ;: 354.6.
References:
CN105439932,2016,A Location in patent:Paragraph 0075; 0076; 0077
![1-Pyrrolidinecarboxylic acid, 3,3'-dithiobis[5-[(dimethylamino)carbonyl]-, 1,1'-bis[(4-nitrophenyl)methyl] ester, (3S,3'S,5S,5'S)-](/CAS/20211123/GIF/936226-36-7.gif)
936226-36-7
5 suppliers
inquiry

96034-64-9
329 suppliers
$8.00/250mg
![1-Pyrrolidinecarboxylic acid, 4-(acetylthio)-2-[(dimethylamino)carbonyl]-, (4-nitrophenyl)methyl ester, (2S,4S)-](/CAS/20200331/GIF/96034-61-6.gif)
96034-61-6
0 suppliers
inquiry

96034-64-9
329 suppliers
$8.00/250mg

506-59-2
527 suppliers
$5.00/5G
![2-Thia-5-azabicyclo[2.2.1]heptane-5-carboxylic acid, 3-oxo-, (4-nitrophenyl)methyl ester, (1S,4S)-](/CAS/20200611/GIF/151072-00-3.gif)
151072-00-3
22 suppliers
inquiry

96034-64-9
329 suppliers
$8.00/250mg
51-35-4
822 suppliers
$6.00/5g

96034-64-9
329 suppliers
$8.00/250mg