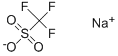
Sodium trifluoromethanesulfonate synthesis
- Product Name:Sodium trifluoromethanesulfonate
- CAS Number:2926-30-9
- Molecular formula:CF3NaO3S
- Molecular Weight:172.06
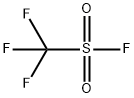
335-05-7
42 suppliers
inquiry

2926-30-9
261 suppliers
$11.00/5g
Yield:2926-30-9 1100 g
Reaction Conditions:
with sodium hydroxide;calcium oxide in water; under 1500.15 - 7500.75 Torr; for 9.5 h;
Steps:
3; 5 Example 3
875 g of a 32.0% aqueous solution of sodium hydroxide, 424 g of calcium oxide and 580 g of high purity water were successively added to a 316L stainless steel reaction vessel, and the temperature was raised to 85 ° C and the mixture was stirred for 3 hours. Then, the trifluoromethane sulfonyl fluoride gas was continuously introduced into the reaction vessel, Controlling the reaction pressure to be between 0.2 MPa and 1.0 MPa; stopping the ventilation when the mass of ventilation is 980 g of trifluoromethanesulfonyl fluoride, the ventilation time is 4 hours, then keeping the reaction for 2.5 hours, then lowering the temperature to 25 DEG C; The filtrate was filtered to obtain an aqueous solution of sodium trifluoromethanesulfonate. The concentration of sodium trifluoromethanesulfonate was 47.55%, pH = 14, F = 7 ppm, and ICP was 0.5 ppm and 0.14 ppm respectively. Fluoride ion concentration <10ppm, Fe <1ppm, Ni <1ppm, indicating that the reaction of 316L stainless steel corrosion, the method greatly reduces the corrosion of equipment, equipment can greatly extend the service life and maintenance costs.
References:
CN105693561,2016,A Location in patent:Paragraph 0028; 0031
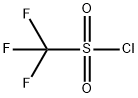
421-83-0
232 suppliers
$22.39/1G

2926-30-9
261 suppliers
$11.00/5g
![Phosphonium, [1-methyl-2-phenyl-2-[[(trifluoromethyl)sulfonyl]oxy]ethenyl]triphenyl-, 1,1,1-trifluoromethanesulfonate (1:1)](/CAS/20211123/GIF/84370-15-0.gif)
84370-15-0
0 suppliers
inquiry
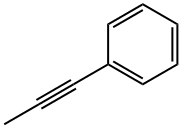
673-32-5
124 suppliers
$11.00/1g

2926-30-9
261 suppliers
$11.00/5g
603-35-0
694 suppliers
$5.00/5G
![Phosphonium, triphenyl[1-[phenyl[[(trifluoromethyl)sulfonyl]oxy]methylene]pentyl]-, 1,1,1-trifluoromethanesulfonate (1:1)](/CAS/20211123/GIF/84370-23-0.gif)
84370-23-0
0 suppliers
inquiry

1129-65-3
70 suppliers
$45.00/50mg

2926-30-9
261 suppliers
$11.00/5g

603-35-0
694 suppliers
$5.00/5G
![Phosphonium, [1-butyl-2-[[(trifluoromethyl)sulfonyl]oxy]-1-hexen-1-yl]triphenyl-, 1,1,1-trifluoromethanesulfonate (1:1)](/CAS/20211123/GIF/84370-17-2.gif)
84370-17-2
0 suppliers
inquiry

1942-46-7
93 suppliers
$6.00/250mg

2926-30-9
261 suppliers
$11.00/5g

603-35-0
694 suppliers
$5.00/5G