
STEAROYL ETHANOLAMIDE synthesis
- Product Name:STEAROYL ETHANOLAMIDE
- CAS Number:111-57-9
- Molecular formula:C20H41NO2
- Molecular Weight:327.55


141-43-5
860 suppliers
$9.00/10g
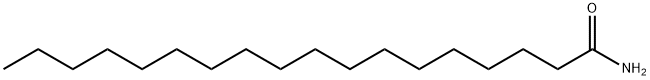
124-26-5
306 suppliers
$14.00/100g

111-57-9
106 suppliers
$49.00/5mg
Yield:111-57-9 74%
Reaction Conditions:
Stage #1:stearamide with triethylamine;methyl chloroformate in dichloromethane at 0 - 20; for 2 h;
Stage #2:ethanolamine in dichloromethane at 0 - 20; for 20 h;
Steps:
N-(2-Hydroxyethyl)stearamide (34)
General procedure: To a mixture of 8-(dimethyl(octyl)silyl)octanoic acid (18a) (4.7 mg, 14.9 nmol) and triethylamine (4.15 μL, 29.8 nmol) in dichloromethane (0.5 mL) was added methyl chloromate (1.80 μL, 29.8 nmol) at 0 °C, and stirred at r.t. for 2 h. Then, ethanolamine was added with stirring at 0 °C, the mixture was stirred at r.t. for 20 h. The title compound was prepared according to the procedure described for 19, starting from compound 33. Yield: 74%. Colorless oil.
References:
Kajita, Daisuke;Nakamura, Masaharu;Matsumoto, Yotaro;Ishikawa, Minoru;Hashimoto, Yuichi;Fujii, Shinya [Bioorganic and Medicinal Chemistry Letters,2015,vol. 25,# 16,art. no. 22735,p. 3350 - 3354] Location in patent:supporting information

141-43-5
860 suppliers
$9.00/10g
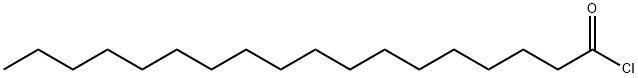
112-76-5
240 suppliers
$40.32/25G

111-57-9
106 suppliers
$49.00/5mg

141-43-5
860 suppliers
$9.00/10g
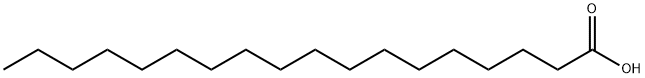
57-11-4
1203 suppliers
$6.00/100g

111-57-9
106 suppliers
$49.00/5mg
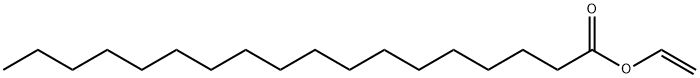
111-63-7
73 suppliers
$27.00/5g

141-43-5
860 suppliers
$9.00/10g

10287-60-2
2 suppliers
inquiry

111-57-9
106 suppliers
$49.00/5mg