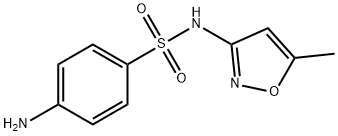
Sulfamethoxazole synthesis
- Product Name:Sulfamethoxazole
- CAS Number:723-46-6
- Molecular formula:C10H11N3O3S
- Molecular Weight:253.28
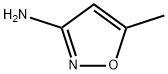
21312-10-7
102 suppliers
$30.00/20mg

723-46-6
614 suppliers
$5.00/5g
Yield:723-46-6 96%
Reaction Conditions:
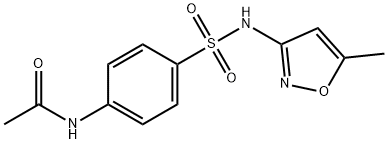
Stage #1: N-acetylsulfamethoxazolewith sodium hydroxide;water at 80; for 1 h;
Stage #2: with acetic acid in water; pH=6;
Steps:
4-Amino-N- (5-methvl-isoxazol-3-vl)-benzenesulphonamide (STX608, XDS01099); The solution of N- (5-methylisoxazol-3-yl)-4-acetamidobenzenesulphonamid (3.4 g, 11.5 mmol) in 10% NaOH solution (15 mL) was stirred at 80°C for 1h, cooled to rt and neutralized to pH 6 with acetic acid. The precipitate was washed with water, dried in vacuo to yield off-white solid (2,8 g, 96%). Mp167-169°C (lit [29], 168-171°C) ; TLC single spot at Rf 0.39 (6% methanol-DCM) ; HPLC purity > 99% (tR 1.6 min in 4% water- methanol) ;'H NMR (400 MHz, CD30D) : S 7.54 (2H, m, ArH), 6.63 (2H, m, ArH), 6.08 (1H, s, ArH), 2.30 (3H, s, CH3) ; FAB-MS 254 (MH+) ; FAB-HRMS calcd for C10H12N303S (MH+) 254.0599, found 254.0605.
References:
WO2005/42513,2005,A1 Location in patent:Page/Page column 89-90
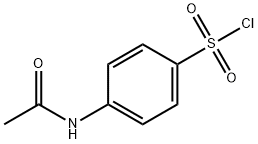
121-60-8
326 suppliers
$10.00/1g

723-46-6
614 suppliers
$5.00/5g

29699-89-6
17 suppliers
inquiry

723-46-6
614 suppliers
$5.00/5g