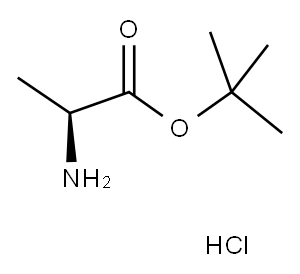
L-Ala-OtBU.HCl synthesis
- Product Name:L-Ala-OtBU.HCl
- CAS Number:13404-22-3
- Molecular formula:C7H16ClNO2
- Molecular Weight:181.66
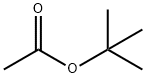
540-88-5
353 suppliers
$21.00/25mL
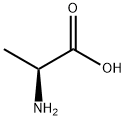
56-41-7
768 suppliers
$5.00/5g

13404-22-3
296 suppliers
$11.19/5G
Yield:-
Reaction Conditions:
with hydrogenchloride;perchloric acid in diethyl ether
Steps:
V.g Preparation of L-alanyl-L-alanine tert-butyl ester, acetate (51)
In a 2 L one-neck flask, L-alanine (6.23 g) is stirred for 4 days with 1.1 L of t-butylacetate at room temperature and perchloric acid (70%, 6.65 mL, 7.7. g). The mixture is concentrated to 1/4 of its original volume, and the residue extracted at 0°-5° C. with 0.5N HCl (4*60 mL). The aqueous phase is immediately neutralized with KHCO3 (solid). The pH is adjusted to 13 with 4N NaOH, and the solution extracted with Et2 O (4*100 mL). The combined organic layers are washed with bicarbonate solution (2*50 mL), dried (MgSO4), and evaporated. The oily residue is dissolved in 10 mL of Et2 O (anh.) and treated with HCl:Et2 O under cooling. The precipitated salt is filtered and dried to yield 4 g of L-alanine-t-butyl ester hydrochloride having a m.p. 167° C.; [α]20 =+177 (c=2 in EtOH).
References:
Merrell Dow Pharmaceuticals Inc. US4730006, 1988, A
![L-Alanine, N-[(phenylmethoxy)carbonyl]-, 1,1-dimethylethyl ester](/CAS/20200611/GIF/50300-96-4.gif)
50300-96-4
0 suppliers
inquiry

13404-22-3
296 suppliers
$11.19/5G
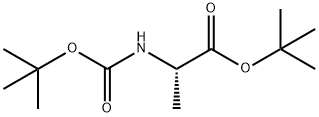
58177-77-8
29 suppliers
inquiry

13404-22-3
296 suppliers
$11.19/5G