
Tetradecanoic acid, 14-bromo-, methyl ester synthesis
- Product Name:Tetradecanoic acid, 14-bromo-, methyl ester
- CAS Number:26825-90-1
- Molecular formula:C15H29BrO2
- Molecular Weight:321.29
![Tetradecanoic acid, 14-[(methylsulfonyl)oxy]-, methyl ester](/CAS/20210305/GIF/934018-81-2.gif)
934018-81-2
0 suppliers
inquiry

26825-90-1
0 suppliers
inquiry
Yield:26825-90-1 100%
Reaction Conditions:
with lithium bromide in acetone;Reflux;
Steps:
Above prepared mesylate (9.15 g, 26.0 mmol) wasdissolved in acetone (230 mE) and lithium bromide (4.50 g,51.8 mmol) was added and the reaction mixture was refluxedovernight. After cooling down solvent was evaporated, ethylacetate (530 mE) was added and the mixture was extractedwith 5% solution of sodium hydrogencarbonate (3x230mE). Combined organic extracts were dried over anhydrousmagnesium sulfate and evaporated to dryness to yield14-bromo-tetradecanoic acid methyl ester as orange oil.Yield: 8.34 g (100%). RF (Si02, dichloromethane/methanol 95:5): 0.90.1H NMR spectrum (300 MHz, CDC13, dH): 3.66(s, 3H); 3.42 (t, J=6.9 Hz, 2H); 2.32 (t, J=7.5 Hz, 2H);1.93-1.80 (m, 2H) 1.69-1.56 (m, 2H); 1.50-1.38 (m, 2H);1.28 (bs, 16H).
References:
US2016/289283,2016,A1 Location in patent:Paragraph 0266; 0267; 0268; 0269

30515-28-7
164 suppliers
$7.00/250mg
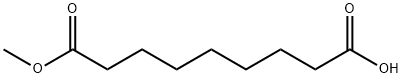
2104-19-0
145 suppliers
$16.00/1g

26825-90-1
0 suppliers
inquiry
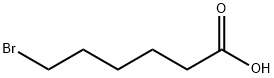
4224-70-8
340 suppliers
$7.00/5g

818-88-2
134 suppliers
$14.00/5g

26825-90-1
0 suppliers
inquiry
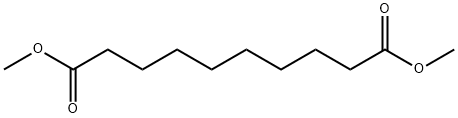
106-79-6
298 suppliers
$6.00/25g

1472-93-1
80 suppliers
$22.00/1g
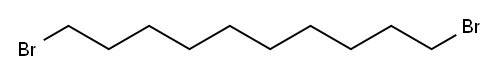
4101-68-2
293 suppliers
$6.00/10g

234082-00-9
82 suppliers
$17.00/100mg

26825-90-1
0 suppliers
inquiry