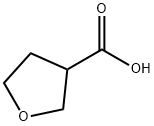
TETRAHYDRO-3-FUROIC ACID synthesis
- Product Name:TETRAHYDRO-3-FUROIC ACID
- CAS Number:89364-31-8
- Molecular formula:C5H8O3
- Molecular Weight:116.12
79710-86-4
80 suppliers
$15.00/1g

89364-31-8
194 suppliers
$8.00/1g
Yield:-
Reaction Conditions:
with oxygen in water at 68 - 125; under 6464.52 Torr; for 0 - 6.53333 h;
Steps:
1; 2; 3; 4; 5; 6; 7; 8
EXAMPLES; The operation of the process of the present invention is further illustrated by the following examples wherein all percentages given are by weight unless otherwise specified. The oxidation experiments were carried out in a stainless steel, 100 ml volume, high pressure autoclave reactor (Autoclave Engineers EZ-Seal) equipped with a high pressure gas manifold inlet system capable of adding oxygen-containing gas streams to the liquid phase reaction medium. The gas stream was added below the liquid level within the reactor near the impeller/stirrer. The stirrer was maintained at 1500 RPM to ensure rapid and even mixing of the oxygen-containing gas stream with the liquid 3-FTHF/solvent medium within the reactor. In order to maintain constant pressure within the reactor body, a high pressure, total reflux column served to vent the gas stream from the reactor body. A back pressure regulator was used to maintain the overall reactor pressure at the desired pressure. The 3-FTHF solution was added to the solvent and oxygen-containing gas stream of the autoclave by means of a high pressure syringe pump to initiate the thermal oxidation reaction. Microliter-sized samples of the liquid phase reaction medium were taken at different time intervals by means of a capillary dip tube maintained in the liquid level of the reactor.In a typical experiment, the reactor was charged with 40 g of water and 12 drops of an internal GC standard, tetramethylene sulfone, and sealed. The desired gas phase composition of oxygen with optional inert gas diluent was flowed through the reactor and the desired reaction pressure set using the back pressure reactor at the exit of the reflux condenser. The reactor was then heated to the desired reaction temperature using a band heater tightly wrapped around the exterior of the reactor body. The stir rate of the solution was set at 1500 RPM to ensure rapid and uniform mixing at all reaction conditions. The pressure was raised to 8.6 bara (125 pounds per square inch-psia) using a pre-determined gas flow composition of O2 and He to give the desired O2 partial pressure. Constant pressure was maintained in the autoclave reactor by the back pressure regulator after the gaseous reactor effluent was cooled to 2° C. in a total reflux-type of stainless steel coiled condenser.After the reactor was heated to the desired temperature, 10.0 g of a 50% solution of 3-FTHF in water quickly was added at high pressure through the syringe pump into the reactor to give a total volume of approximately 50 mls solution, which defined time=0 for the kinetic experiments. Each reaction typically was run for 5 to 6 hours, samples were taken periodically using the dip tube in the reactor and were subsequently analyzed by gas chromatography (GC). In one experiment, off gases were collected in a gas sample bomb and analyzed for CO2 using a different GC, which had been calibrated for CO2. Negligible amounts of CO2-were observed at all reaction times, confirming that combustion of feed and product were not occurring during the thermal oxidation reaction.; Example 1; 3-FTHF was oxidized over a period of 5 hours at 100° C. and 8.6 bara (125 psia) overall pressure (5.0 bara {72.5 psia} O2 and 3.6 bara {52.5 psia} He) according to the procedure described above. The course of the reaction was observed by periodically sampling the reaction mixture and analyzing for 3-FTHF. The results are shown in Table I wherein Time is the time (minutes) of the reaction measure as described above and 3-FTHF is the normalized micromoles of unreacted 3-FTHF present in the reaction mixture.; Example 2; The procedure of Example 1 was repeated except that after 150 minutes of reaction time of a second aliquot of 10.0 grams of 50% aqueous 3-FTHF solution was pumped into the autoclave reactor. The course of the reaction was observed by periodically sampling the reaction mixture and analyzing for 3-FTHF. The results are shown in Table II wherein Tim is the time (minutes) of the reaction measured as described above and 3-FTHF is the normalized micromoles of unreacted 3-FTHF present in the reaction mixture.; Examples 3-5; The procedure of Example 1 was repeated using different reaction temperatures as shown in Table III wherein Temp is the temperature (° C.) at which the experiment was carried out and Time has the meaning given above in Example 1. In these examples, the O2 pressure was maintained constant at 8.6 bara (125 psia) at a total reaction pressure of 8.6 bara1(25 psia). The samples taken periodically were analyzed for 3-FTHF and 3-THFA. Table III also reports the Conversion of 3-FTHF and the Selectivity to 3-THFA at each point of Time. Conversion is the percent conversion of FTHF to all products: Selectivity is the percent selectivity to THFA defined as; Examples 6-8; The procedure of Example 1 was repeated at 100° C. and 8.6 bara (125 psia) overall pressure except that different oxygen partial pressures were used as shown in Table IV wherein O2 Press is the oxygen partial pressure (bara-psia) at which the experiment was carried out and Time has the meaning given above in Example 1. The samples taken periodically were analyzed for 3-FTHF and 3-THFA. Conversion and Selectivity have the meanings provided in Examples 3-5.; Example 9; The basic procedure of Example 1 was repeated except that the starting material was 75 g of a 50% aqueous 3-FTHF solution and the oxidation was carried out over a time of 3 hours. The results are shown in Table V wherein Time, Conversion and Selectivity have the meanings provided in the preceding examples.
References:
Eastman Chemical Company US6881851, 2005, B1 Location in patent:Page/Page column 3-5
488-93-7
375 suppliers
$5.00/250mg

89364-31-8
194 suppliers
$8.00/1g

130193-90-7
0 suppliers
inquiry

89364-31-8
194 suppliers
$8.00/1g

488-93-7
375 suppliers
$5.00/250mg

89364-31-8
194 suppliers
$8.00/1g

1679-47-6
114 suppliers
$9.00/1g
1445651-64-8
7 suppliers
inquiry

89364-31-8
194 suppliers
$8.00/1g